This is a reprint of a news release posted by the World Meteorological Organization on Nov 20, 2012.
Geneva, 20 November (WMO) – The amount of greenhouse gases in the atmosphere reached a new record high in 2011, according to the World Meteorological Organization. Between 1990 and 2011 there was a 30% increase in radiative forcing – the warming effect on our climate – because of carbon dioxide (CO2) and other heat-trapping long-lived gases;
Since the start of the industrial era in 1750, about 375 billion tonnes of carbon have been released into the atmosphere as CO2, primarily from fossil fuel combustion, according to WMO’s 2011 Greenhouse Gas Bulletin, which had a special focus on the carbon cycle. About half of this carbon dioxide remains in the atmosphere, with the rest being absorbed by the oceans and terrestrial biosphere.
“These billions of tonnes of additional carbon dioxide in our atmosphere will remain there for centuries, causing our planet to warm further and impacting on all aspects of life on earth,” said WMO Secretary-General Michel Jarraud. “Future emissions will only compound the situation.”
“Until now, carbon sinks have absorbed nearly half of the carbon dioxide humans emitted in the atmosphere, but this will not necessarily continue in the future. We have already seen that the oceans are becoming more acidic as a result of the carbon dioxide uptake, with potential repercussions for the underwater food chain and coral reefs. There are many additional interactions between greenhouse gases, Earth’s biosphere and oceans, and we need to boost our monitoring capability and scientific knowledge in order to better understand these,” said Mr Jarraud.
“WMO’s Global Atmosphere Watch network, spanning more than 50 countries, provides accurate measurements which form the basis of our understanding of greenhouse gas concentrations, including their many sources, sinks and chemical transformations in the atmosphere,” said Mr Jarraud.
The role of carbon sinks is pivotal in the overall carbon equation. If the extra CO2 emitted is stored in reservoirs such as the deep oceans, it could be trapped for hundreds or even thousands of years. By contrast, new forests retain carbon for a much shorter time span.
The Greenhouse Gas Bulletin reports on atmospheric concentrations – and not emissions - of greenhouse gases. Emissions represent what goes into the atmosphere. Concentrations represent what remains in the atmosphere after the complex system of interactions between the atmosphere, biosphere and the oceans.
CO2 is the most important of the long-lived greenhouse gases – so named because they trap radiation within the Earth’s atmosphere causing it to warm. Human activities, such as fossil fuel burning and land use change (for instance, tropical deforestation), are the main sources of the anthropogenic carbon dioxide in the atmosphere. The other main long-lived greenhouse gases are methane and nitrous oxide. Increasing concentrations of the greenhouse gases in the atmosphere are drivers of climate change.
The National Oceanic and Atmospheric Administration’s Annual Greenhouse Gas Index, quoted in the bulletin, shows that from 1990 to 2011, radiative forcing by long-lived greenhouse gases increased by 30%, with CO2 accounting for about 80% of this increase. Total radiative forcing of all long-lived greenhouse gases was the CO2 equivalent of 473 parts per million in 2011.
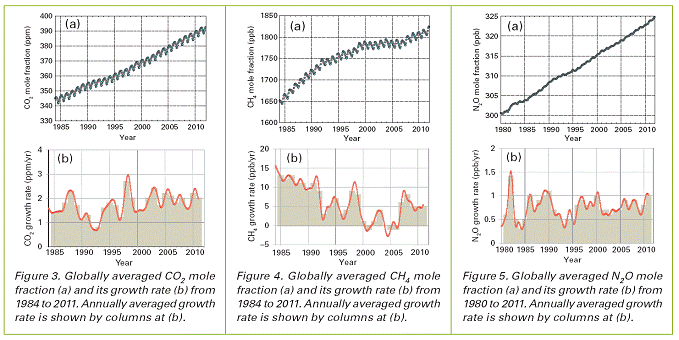
Carbon dioxide is the single most important greenhouse gas emitted by human activities. It is responsible for 85% of the increase in radiative forcing over the past decade. According to WMO’s bulletin, the amount of CO2 in the atmosphere reached 390.9 parts per million in 2011, or 140% of the pre-industrial level of 280 parts per million.
The pre-industrial era level represented a balance of CO2 fluxes between the atmosphere, the oceans and the biosphere. The amount of CO2 in the atmosphere has increased on average by 2 parts per million per year for the past 10 years.
Methane is the second most important long-lived greenhouse gas. Approximately 40% of methane is emitted into the atmosphere by natural sources (e.g., wetlands and termites), and about 60 % comes from activities like cattle breeding, rice agriculture, fossil fuel exploitation, landfills and biomass burning. Atmospheric methane reached a new high of about 1813 parts per billion (ppb) in 2011, or 259% of the pre-industrial level, due to increased emissions from anthropogenic sources. Since 2007, atmospheric methane has been increasing again after a period of levelling-off with a nearly constant rate during the last 3 years.
Nitrous oxide is emitted into the atmosphere from both natural (about 60%) and anthropogenic sources (approximately 40%), including oceans, soil, biomass burning, fertilizer use, and various industrial processes. Its atmospheric concentration in 2011 was about 324.2 parts per billion, which is 1.0 ppb above the previous year and 120% of the pre-industrial level. Its impact on climate, over a 100 year period, is 298 times greater than equal emissions of carbon dioxide. It also plays an important role in the destruction of the stratospheric ozone layer which protects us from the harmful ultraviolet rays of the sun.

Source: 2011 Greenhouse Gas Bulletin
The WMO Secretariat prepares and distributes the annual Greenhouse Gas Bulletin in cooperation with the World Data Centre for Greenhouse Gases at the Japan Meteorological Agency and the Global Atmosphere Watch Scientific Advisory Group for Greenhouse Gases, with the assistance of the NOAA Earth System Research Laboratory.
The Intergovernmental Panel on Climate Change defines radiative forcing as a measure of the influence a factor has in altering the balance of incoming and outgoing energy in the Earth-atmosphere system and is an index of the importance of the factor as a potential climate change mechanism. Radiative forcing values are often expressed in watts per square meter.
Posted by John Hartz on Thursday, 22 November, 2012
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |