How do we know more CO2 is causing warming?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
An enhanced greenhouse effect from CO2 has been confirmed by multiple lines of empirical evidence. |
|||||||
Climate Myth...
Increasing CO2 has little to no effect
"While major green house gas H2O substantially warms the Earth, minor green house gases such as CO2 have little effect.... The 6-fold increase in hydrocarbon use since 1940 has had no noticeable effect on atmospheric temperature ... " (Environmental Effects of Increased Atmospheric Carbon Dioxide)
At-a-glance
To make a statement like, "minor greenhouse gases such as CO2 have little effect", is to ignore 160 years of science history. So let's look at who figured out the heat-trapping properties of carbon dioxide and when.
Experiments involving various gas mixtures had demonstrated the heat-trapping properties of water vapour, CO2 and methane in the 1850s. But those effects were yet to be quantified - there were no meaningful numbers. It was to be another 40 years before that happened.
Swedish scientist Svante Arrhenius (1859-1927) was the person who crunched the numbers. The results were presented in a remarkable paper, "On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground", in 1896.
The many calculations in the 1896 paper include estimates of the amounts of CO2 increase or decrease required to drive the climate into a different state. One example used was the Hothouse climate of the Cenozoic, around 50 million years ago. Another was the glaciations of the last few hundred millennia.
To get a temperature rise of 8-9°C in the Arctic, Arrhenius calculated that CO2 levels would have to increase by 2.5 to 3 times 1890s levels. To lower the temperature 4–5°C to return to glacial conditions, he calculated a drop in CO2 was needed of 0.62-0.55 times 1890s levels.
We know CO2 levels in the 1890s from ice-core data. They were around 295 ppm. Let's do the sums. A reduction factor of 0.55 to 0.62 on 295 ppm gives 162.2-183.9 ppm. Modern ice-core measurements representing the past 800,000 years show that in glacial periods, CO2 levels fell to 170-180 ppm.
What we now know due to additional research since 1896 when Arrhenius worked on this, is that CO2 was an essential 'amplifying feedback'. That means changes triggered by long term, cyclic variations in Earth's orbit cause warming or cooling and CO2 release or entrapment in turn. Those changes in CO2 levels affected the strength of Earth's greenhouse effect. Changes in the strength of the greenhouse effect then completed the job of pushing conditions from interglacial to glacial - or vice-versa.
Arrhenius also made an important point regarding water vapour: "From observations made during balloon voyages, we know also that the distribution of the aqueous vapour may be very irregular, and different from the ideal mean distribution." This statement holds true today: water vapour is a greenhouse gas but because water exists in gas, liquid and solid forms in the atmosphere, it is continually cycling in and out of the air. It is distributed in a highly uneven fashion and is uncommon in the upper atmosphere. That's where it differs from CO2.
Once CO2 is up there, it's up there for a long time. As a consequence it has a pretty even distribution: 'well-mixed' is the term. As Arrhenius quantified all that time ago, once it's up there it constantly absorbs and re-radiates heat in all directions. That's why dumping 44 billion tons of it into our atmosphere in just one year (2019 - IPCC Sixth Assessment Report 2022) is a really bad idea.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
Good scientific theories are said to have ‘predictive power’. In other words, armed only with a theory, we should be able to make predictions about a subject. If the theory’s any good, the predictions will come true.
Here’s an example: when the Periodic Table of the chemical elements was proposed in 1869, many elements were yet to be discovered. Using the theory behind the Periodic Table, the Russian chemist Dmitri Mendeleev was able to predict the properties of germanium, gallium and scandium prior to their discovery in 1886, 1875 and 1879 respectively. His predictions were found to be correct.
The effect on Earth's greenhouse effect of adding man-made CO2 is predicted in the theory of greenhouse gases. This theory was first proposed by Swedish scientist Svante Arrhenius in 1896, based on earlier work by Fourier, Foote and Tyndall. Many scientists have refined the theory since Arrhenius published his work in 1896. Nearly all have reached the same conclusion: if we increase the amount of greenhouse gases in the atmosphere, the Earth will warm up.
Where there is less agreement is with respect to the exact amount of warming. This issue is called 'climate sensitivity', the amount the temperatures will increase if CO2 is doubled from pre-industrial levels. Climate models have predicted the least temperature rise would be on average 1.65°C (2.97°F) , but upper estimates vary a lot, averaging 5.2°C (9.36°F). Current best estimates are for a rise of around 3°C (5.4°F), with a likely maximum of 4.5°C (8.1°F). A key reason for this range of outcomes is because of the large number of potential climate feedbacks and their variable interactions with one another. Put simply, some are much better understood than others.
What Goes Down…
The greenhouse effect works like this: Energy arrives from the sun in the form of visible light and ultraviolet radiation. The Earth then emits some of this energy as infrared radiation. Greenhouse gases in the atmosphere 'capture' some of this heat, then re-emit it in all directions - including back to the Earth's surface.
Through this process, CO2 and other greenhouse gases keep the Earth’s surface 33°Celsius (59.4°F) warmer than it would be without them. We have added 42% more CO2, and temperatures have gone up. There should be some evidence that links CO2 to the temperature rise.
So far, the average global temperature has gone up by more than 1 degrees C (1.9°F):
"According to an ongoing temperature analysis led by scientists at NASA’s Goddard Institute for Space Studies (GISS), the average global temperature on Earth has increased by at least 1.1° Celsius (1.9° Fahrenheit) since 1880. The majority of the warming has occurred since 1975, at a rate of roughly 0.15 to 0.20°C per decade."
The temperatures are going up, just like the theory predicted. But where’s the connection with CO2, or other greenhouse gases like methane, ozone or nitrous oxide?
The connection can be found in the spectrum of greenhouse radiation. Using high-resolution FTIR spectroscopy, we can measure the exact wavelengths of long-wave (infrared) radiation reaching the ground.
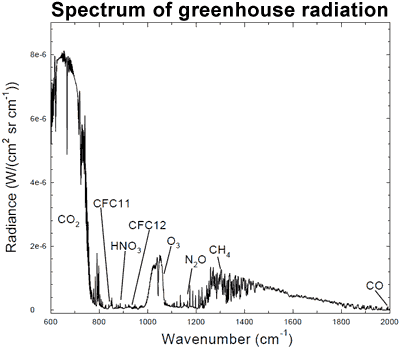
Figure 1: Spectrum of the greenhouse radiation measured at the surface. Greenhouse effect from water vapour is filtered out, showing the contributions of other greenhouse gases (Evans 2006).
Sure enough, we can see that CO2 is adding considerable warming, along with ozone (O3) and methane (CH4). This is called surface radiative forcing, and the measurements are part of the empirical evidence that CO2 is causing the warming.
...Must Go Up
How long has CO2 been contributing to increased warming? According to NASA, “Two-thirds of the warming has occurred since 1975”. Is there a reliable way to identify CO2’s influence on temperatures over that period?
There is: we can measure the wavelengths of long-wave radiation leaving the Earth (upward radiation). Satellites have recorded the Earth's outgoing radiation. We can examine the spectrum of upward long-wave radiation in 1970 and 1997 to see if there are changes.
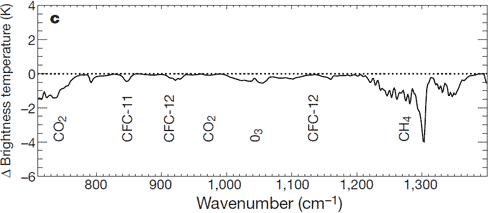
Figure 2: Change in spectrum from 1970 to 1996 due to trace gases. 'Brightness temperature' indicates equivalent blackbody temperature (Harries et al. 2001).
This time, we see that during the period when temperatures increased the most, emissions of upward radiation have decreased through radiative trapping at exactly the same wavenumbers as they increased for downward radiation. The same greenhouse gases are identified: CO2, methane, ozone and so on.
The Empirical Evidence
As temperatures started to rise, scientists became more and more interested in the cause. Many theories were proposed. All save one have fallen by the wayside, discarded for lack of evidence. One theory alone has stood the test of time, strengthened by experiments.
We have known CO2 absorbs and re-emits longwave radiation, since the days of Foote, Tyndall and Arrhenius in the 19th Century. The theory of greenhouse gases predicts that if we increase the proportion of greenhouse gases, more warming will occur.
Scientists have measured the influence of CO2 on both incoming solar energy and outgoing long-wave radiation. Less longwave radiation is escaping to space at the specific wavelengths of greenhouse gases. Increased longwave radiation is measured at the surface of the Earth at the same wavelengths.
Last updated on 16 July 2023 by John Mason. View Archives































 Arguments
Arguments






































Ok thanks Scaddenp
Just to clarify
1. Yes that is what I meant, I used a line analogy rather than a photon traveling in a straight line.
2. If I read you correct then, when a photon strikes a CO2 molecule it re-radiates exactly the same amount of energy without losing energy to heat, either by heating itself or surrounding molecules.
3. Yes I understand that the only way heat leaves earth is by radiation, but is it not greatly diminished in the band that CO2 responds to.
4. My question is, does air, primarily O2 and N2 give off Long wave radiation when it's heated. What is radiating the heat at the top of storm clouds?
5. Maybe a better way to ask the question, what percentage of the total heat budget is in the long wave radiation band that CO2 responds to. If none of that band were allowed to escape, then the earth would have to heat to expel more at the frequency bands that gets through.
I'm new at this but enjoy reading all the discussions, thanks
Just_Curious - "...when a photon strikes a CO2 molecule it re-radiates exactly the same amount of energy without losing energy to heat, either by heating itself or surrounding molecules." No.
Checking the numbers, that is not actually the case. Relaxation to emission of a radiation excited CO2 molecule takes about 10-6 seconds, whereas at sea level pressure a CO2 molecule will undergo a collision about every 10-9 seconds. There are therefore about 1000 collisions exchanging energy at sea level before that molecule can emit.
What happens is that the air mass containing the absorbing CO2 (including O2, N2, etc.) molecule warms due to the absorbed energy, and (statistially) some other GHG molecules will radiate at some frequency in their spectra in a matching amount, due to the changed temperature of the air mass. Not the same molecule in 999/1000 cases.
Just-Curious @149 asks:
Answering this question literally, the answer is much, much greater than 150 kilometers. That is, there is essentially zero chance that such a line will strike a CO2 molecule before exiting the Earth's atmosphere.
This answer is very different from that by scaddenp @ 150, but that is because scaddenp made the "mistake" of answering the question he thought you intended rather than the question you asked. As a result he gave an approximate answer to the question, how long would an IR photon travel before interacting with a CO2 molecule if the photon was at the right wavelength, and if the CO2 molecule was in the right excitation state.
Lines do not have electrical fields. Therefore to come into contact with a CO2 molecule, it would need to strike the nuclei of the one of the three atoms in the molecule. As you have probably heard somewhere, atoms (and molecules even more so) are mostly empty space. The nuclei are very small relative to the size of the electron shells. So small that neutrons (which because they have no charge, interact only with the nuclei of atoms) have a mean free-path length of 1.91 cm when travelling through uranium oxide. In contrast, of all the CO2 in the atmosphere were to be reduced to a solid (dry ice) layer evenly covering the surface of the Earth, it would only be 0.1 cm thick. That is, you would have to increase the Earth's CO2 concentration by a factor of 19, or the thickness of the atmosphere by a similar amount, to have an approximately 50/50 chance that a neutron traveling vertically from the surface would strike a CO2 molecule. And, of course, lines are much thinner than neutrons, and so have an even lower chance of striking a molecule.
I note this not just from empty pedantry, or an inordinate love of trivia. It is to emphasize that the interactions between light and CO2 molecules are mediated by the electromagnetic force. Because of that, the IR radiation must have exactly the right wavelength if it is to interact. If it does not, if its wavelength is 18 microns rather than 15 microns, for example, it will breeze past all the CO2 in the atmosphere with no effect. Even if the wavelength is very close to 15 microns, the CO2 molecule has to be traveling in the right direction at the right speed (so that the doppler shift will result in the correct resonance), the and the molecule has to be at the correct excitation state, and so though a number of other factors. If not, the IR radiation will not be absorbed, but simply continue on its way. Consequently, for most IR photons, they will travel through the atmosphere without significant interaction with CO2. But at the crucial wavelength, their mean free-path length is quite short (and at 15 microns, is very close to 3 meters).
Just_Curious @151, KR @152 makes a very astute point.
The result of the fact that most energy absorbed as IR radiation is redistributed to the adjacent atmosphere as some for of kinetic energy via collisions is that the IR radiation from any level of the atmosphere is set the temperature at that level of the atmosphere, plus the radiation not absorbed by that level of the atmosphere. When the radiation leaving the top of that atmosphere is calculated on that principle, with a suitably high resolution, the result is a stunningly accurate prediction by the models of the observed radiation. Any such calculation shows that the greatest impact on outgoing radiation in terms of percentatage of radiation blocked at a given wavelength, is the impact of CO2; and will also show that the active wavelengths of CO2 coincide with the peak IR radiation from the Earth:
(In this graps, the strong spike around a wavenumber of 600 is CO2. The smaller spike around 1100 is ozone. Methane is responsible for the effect around 1300-1400, with H2O responsible for the rest of the reduction in OLR, which is shown by the red shaded region.)
The result is that CO2 is by far the strongest of the IR active gases, with the exception of water vapour. It also means that the greenhouse effect of CO2 is not blocked by that of water vapour.
I recomend that you read my post discussing the basics of the greenhouse effect to help get clear in your mind the relevant facts.
Just_Curious@151
Your questions are quite clear and logical. One way to better understand these things, if you are not an expert yet, is to simplify the 'experiment' you are thinking about. For example:
Let's say we have an atmosphere of nitrogen and some CO2 only. Looking at a layer near the surface (say a hundred meters) in isolation (as if there is nothing above it), how would we characterize (a) the absorption of radiation from the surface and (b) emission at the top? (With the same pressure and temperature, of course.)
If you can explain that, you are already something of an expert, and the rest of it is much easier to follow.
http://www.principia-scientific.org/Current-News/new-discovery-nasa-study-proves-carbon-dioxide-cools-atmosphere.html
how does this square with what you have here?
Old Engineer,
One of the basic predictions of Climate Theory is that CO2 will warm the lower atmosphere and warm the stratosphere. The measurement of this effect is one of the long term successes of Climate Science. Your reference describes the absorption of a small amount of energy from a solar flare in the stratosphere that is then radiated back into space as was long ago predicted by Climate Theory.
Your source states at the end "Some diehard climate alarmists will still say that in the lower atmosphere the action of carbon dioxide is reversed", acknowledging that this is the accepted effect.
If you read the "start here" button at the top of the page it will explain this and many other basics to you.
Typo: my previous post should say "warm the lower atmosphere and cool the stratosphere"
Let's take a look at a small scale model. As everybody knows if you cover a greenhouse frame with clear plastic it quickly gets much hotter inside than outside the greenhouse on a sunny day. Yet if you cover the frame in shade cloth which is perforated, the temperature in the greenhouse goes down in full sunlight. The cloth helps retain some warmth during the night, thus helping to stabilize the extremes. This is a typical greenhouse effect model and is in fact the reason why greenhouses and shadehouses are so popular in backyards. It is also the reason why shade cloth is so popular in large car parks.
Now, consider the following experiment. I have two greenhouses completely covered with clear plastic and both are in full sunlight, out in the open, and side by side, on the same day. I extract all of the air out of the first greenhouse and then pump it full of CO2. I do nothing to the air inside the second greenhouse. Question: Will greenhouse 1 get any hotter than greenhouse 2?
Skinny_Pete...
1) You should read all 3 levels of the article you're commenting on first.
2) You should understand the difference between a greenhouse and the greenhouse effect.
3) You should read the comments policy for SkS before you make another comment.
Now, to answer your question. Yes. Greenhouse 1 will get hotter than greenhouse 2.
Skinny_Pete...
And here is your experiment performed (for all intents and purposes).
Thanks for the brush off. I disagree with the You Tube video. I have tried this experiment numerous times in sunlight and the temperature has always stayed the same in both greenhouses. Clearly many other factors are needed to make the temperature go up, e.g. smoke, dust, pollen, pollutants, water vapour etc.
[JH] Please keep it civil.
Please note that posting comments here at SkS is a privilege, not a right. This privilege can and will be rescinded if the posting individual continues to treat adherence to the Comments Policy as optional, rather than the mandatory condition of participating in this online forum.
Skinny_Pete @159 & 162:
1) With regard to your experiment, nearly all incoming solar radiation is in the visible wavelengths. CO2 does not trap energy in visible wavelengths, and moreover, the ground is a good absorber in the IR wavelengths at which CO2 is a good absorber. Therefore increasing the CO2 in a small box will not increase the temperature of the box in sunlight appreciably due to traping more incoming. Further, due to the small size of the box, the normal motion of atmospheric particles within the box will keep the entirety of the box at essentially the temperarature of the ground on which it is located (or the floor of the box). Therefore any excess energy trapped by CO2 in the box from outgoing IR radiation will be replaced by an equal amount of outgoing IR energy radiated by the CO2. Therefore, and contrary to Rob Honeycutt, the temperature will not be appreciably different in either case. (I have seen several videos of this experiment done, with some showing a slightly higher temperature for the box containing CO2, and some showing the reverse. Both differences are down to errors in the conducting of the experiment.)
2) I am dubious about the experiment shown in the video linked by Rob Honeycutt. The two bottles with stoppers and thermometers installed are essentially air tight. Therefore, plausibly, the CO2 released in the bottle will raise the pressure in the bottle, thereby increasing temperatures. I do not see how this contaminating effect is controlled for, so I consider the results to be dubious at best.
3) Having said that, if the CO2 could be generated seperately and then poured into the bottle before sealing, and then the bottle left to sit till it was at room temperature before turning on the light, you would then have a far better experimental design than typically seen. Specifically, because the light shines in from the side and all sides are transparent, visible light for the most part will shine straight through the bottle without warming, so that IR radiation absorbed by the CO2 would in fact be additional warming. The amount of additional IR radiation absorbed is likely to be small, however, so I am not convinced that the additional warming would be appreciable relative to experimental error.
4) I have given considerable thought as to how to perform the experiment you are interested in correctly. It would be difficult, however, and require maintaining a vacumn in parts of the experiment for the duration of the experiment, so I don't expect to see it done correctly on Youtube any time soon.
5) Such experiments are, however, entirely beside the point. Simple radiation models have shown the ability to predict upward and downward radiation in the Earth's atmosphere with stunning accuracy. Here, for example, is a comparison between predicted and observed upward IR radiation at a site in Texas from 1970:
And here is a comparison of measured and predicted total Outgoing Longwave Radiation for 134,862 observations over a wide range of atmospheric conditions (both as to temperature and latitude) reported in 2008:
These are models of radiation only. They involve no problems with chaos theory, and no problems with performance. It is the output of models like these that predict the greenhouse effect, and that the greenhouse effect is not saturated. The greenhouse effect has been observationally proved to exist, as much as such a term is ever appropriate in science.
Isn't there a "But I built this box/bottle/aquarium experiment, and it proves that . . ." myth somewhere in the list?
Skinny_Pete... You seem to have failed on my other suggestions of reading the relevant material presented in the articles above, and the suggestion that you read the comments policy. I resubmit both of those suggestions to you.
The fact is, the radiative properties of CO2 are extremely well established physics. I can accept Tom's sense that the bottle experiment is not a well controlled one, but you can see the same effects at work with a thermal camera set up. Here.
Question. ... will outgoing LWIR decrease as co2 increases? ???.... producing the greenhouse effect?
Donny @166, increasing the CO2 content of the atmosphere initially decreases the outgoing LWIR. Changes must then occur at the Earth's surface or in the atmosphere to either decrease incoming SW radiation, or increase outgoing LWIR radiation until the two again match, reestablishing equilbrium. Until that occurs, the imbalance will result in additional energy being stored in the Earth's surface systems which will result in rising temperatures. Further, raising surface temperatures is the simplest and most direct means restore the balance. Further, for any other change than raising temperatures to occur, some physical change at the Earth's surface must occur to drive that change, and as that physical change is driven by the imbalance (otherwise the Earth would not reestablish equilibrium), the physical change that drives the other changes will be a change in temperature. Ergo, increasing CO2 will raise temperatures. Tracing the most obvious physical pathways show that it will rise by a significant amount (as do other direct observations, and the paleo record).
In an ever increasing co2 environment shouldn't the OLWIR decrease as the co2 increases?
Especially if you do find rising global temperatures?
Donny @168 and 169, increasing CO2 decreases outgoing IR radiation all else being equal. Increasing surface temperatures increases outgoing IR radiation all else being equal.
Moderator's Comment
Please refrain from responding to Donny's future posts until a Modrator has had time to review their content. Given Donny's propensity to repeatedly violate the SkS Comments Policy, his future posts are likely to be deleted. If they are, your responses to them will be deleted as well.
Moderator's Comment
Donny:
Please note that posting comments here at SkS is a privilege, not a right. This privilege can and will be rescinded if the posting individual continues to treat adherence to the Comments Policy as optional, rather than the mandatory condition of participating in this online forum.
Moderating this site is a tiresome chore, particularly when commentators repeatedly submit offensive, off-topic posts or intentionally misleading comments and graphics or simply make things up. We really appreciate people's cooperation in abiding by the Comments Policy, which is largely responsible for the quality of this site.
Finally, please understand that moderation policies are not open for discussion. If you find yourself incapable of abiding by these common set of rules that everyone else observes, then a change of venues is in the offing.
Please take the time to review the policy and ensure future comments are in full compliance with it. Thanks for your understanding and compliance in this matter, as no further warnings shall be given.
John.... I am not sure what I said that violated the policy???? Can you be more specific so I know?
I am trying to figure out why the OLWIR would not be acting like it should. ... if that is even the case.... is that wrong to do?? I am confused. Why do you say I have repeatedly violated?
Donny, the OLWIR radiation is acting as it should, ie, as a function of surface temperature and greenhouse gas concentrations. You have provided no evidence to the contrary. Indeed, the TOA energy imbalance that is demonstrated by the rising Ocean Heat Content shows the current OLWIR radiation to have increased significantly less than would be expected from the increased surface temperature alone. Indeed, without a decrease of OLWIR due to CO2 (and other greenhouse gases), we would expect a negative imbalance (oceans loosing heat) due to the rise in surface temperatures alone.