OA not OK part 2: Thermodynamic duo
Posted on 5 July 2011 by Doug Mackie
This post is number 2 in a series about ocean acidification. Other posts: Introduction , 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
Welcome to the second post in our series about ocean acidification. In the first post we introduced Equation 1 (shown again below) for the formation of calcium carbonate and showed that the formation of calcium carbonate shells is a source of CO2, not a sink for CO2.
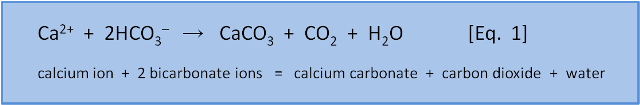
We noted that most chemical reactions can go both forward or backwards and that we could use thermodynamics to predict the direction.
In fact, most reactions go in both directions at once but there is usually a more favoured direction. Consider a dinner party with 6 people. There are 5 people on one side of the table and 1 person on the other. Each side starts with a bowl of peanuts and they begin to throw them at each other. At any one time a few peanuts will be in flight, most of them coming from the side with 5 people. However, it is plain that very quickly almost all of the peanuts will end up on the side with 1 person. At this point, the side with 5 people can only throw peanuts as quickly as the lone peanut pitcher sends them over. An equilibrium has been reached. The number of peanuts on either side does not change even though a few individual peanuts swap sides.
Chemical reactions also proceed until equilibrium is reached. That is, the reaction proceeds until the forward reaction (the reaction on the left side of the equation) and the backwards reaction (the reaction on the right side of the equation) occur at the same rate. For most reactions, one side of an equation is vastly (by a factor of thousands or millions) more favoured than the other and for convenience chemists often write a single arrow to show the favoured direction.
Thermodynamics is based on energy differences and tells us if a reaction occurs under the given conditions. Chemists use the word spontaneous to describe a reaction that occurs without outside intervention. For example, ice melting at room temperature is spontaneous, while liquid water freezing at room temperature is not spontaneous.
Millions of experiments - like ice melting - have been done and the data has allowed chemists to calculate a change in energy content for every type of chemical reaction. It is, of course, complex and there are many considerations. Nevertheless these energy calculations show – and experiments confirm – that calcium and bicarbonate ions react according to Equation 1 (shown above).
Thermodynamics also tells us that the reaction in equation 1 is spontaneous under the conditions in the surface oceans. That is, marine organisms like corals and shellfish are able to extract bicarbonate ions from seawater to make their shells or skeletons. However, as we will see in a later post, those conditions can be changed so that the reverse reaction happens, causing the calcium carbonate to dissolve:

Equation 4 is just Equation 1 running in the reverse direction. This is what takes place when limestone rocks are weathered by the action of rain and air. It is no surprise, therefore, that the most abundant ions in most river waters, calcium (Ca2+) and bicarbonate (HCO3–), are derived from weathering. (Post 6 discusses weathering in detail).
Thus equations 1 and 4, gentle readers, explain the formation of carbonates and rock weathering. They also explain the time scales on which these reactions occur, oceanic control of atmospheric CO2, and why acidification is happening in our ocean. We will cover each of these in the coming series of posts. In the next post we cover why it is easy to use Equation 1 to make shells.
Written by Doug Mackie, Christina McGraw , and Keith Hunter . This post is number 2 in a series about ocean acidification. Other posts: Introduction , 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.































 Arguments
Arguments

























 0
0  0
0 second summary post
second summary post







Comments