OA not OK part 9: Henry the 8th I was (*)
Posted on 23 July 2011 by Doug Mackie
This post is number 9 in a series about ocean acidification. Other posts: Introduction , 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
Welcome to the 9th post in our series about ocean acidification. How does CO2 get into the ocean? We saw back in post 5 that the oceans contain 50 times more carbon than the atmosphere. Is it possible that instead the oceans are actually the source of CO2 in the atmosphere, not fossil fuels? The relevant equation (below) is the equilibrium of CO2 in the atmosphere (or gas phase) and CO2 absorbed by the ocean:

In post 5 with equations 7-9 we showed what can happen once CO2 is absorbed, but Eq. 13 (in the left to right direction) is the process of getting the CO2 into the seawater in the first place. The reverse process (right to left) is called degassing. Familiar examples of degassing are carbonated beverages, like beer and fizzy drinks, going flat as they release CO2 to the atmosphere. To follow and predict what happens we need to know some gas laws.
The first is Dalton's law of partial pressures. Dalton's law is that the total pressure exerted by a gas mixture is the sum of the pressure exerted by each component. For example the pressure exerted by a container of compressed air is the sum of the pressure exerted by the oxygen + the pressure exerted by the nitrogen + the pressure exerted by the argon + the pressure exerted by the carbon dioxide + all the other components. The pressure exerted by a given component is called its partial pressure.
The other gas law we need to know is Henry's law of gas solubility. Henry's law is sort of an extension of Dalton's and says that if you have a liquid in equilibrium with the gas above it, then the amount of gas that is absorbed in the liquid is directly proportional to the partial pressure of the gas. That is, if you take a vessel half full of water and double the amount of air by pressurising the air space, then twice as much of each component in the air is absorbed.
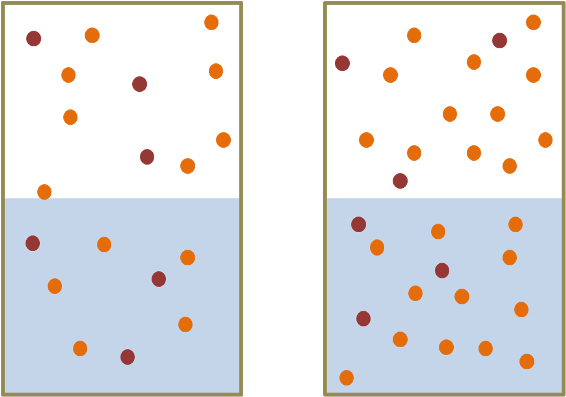
Henry's Law. The more molecules of a gas (i.e., the greater the partial pressure) then the more of that gas that dissolves in the water. In the headspace above the water the number of orange dots has increased from left to right. Henry's Law tells us that therefore the number of orange dots in the water also increases from left to right. (See also this clip at YouTube).
A Henry's Law coefficient, KH expresses the equilibrium ratio between the two sides of Eq. 13. However, a critical point to note is that the ocean is NOT in such equilibrium with the atmosphere. (We will return to this in a later post). Calculations are complicated because of biological activity and because; as we have seen in post 5, as soon as CO2 is absorbed then it forms carbonic acid. This disturbs the equilibrium in each of Equations 7-9, which in turn disturbs the equilibrium in Eq. 13. However these gas laws tell us that the concentration (really we should say partial pressure) of CO2 in both the atmosphere and the oceans are directly related. That is, a CO2 increase in the atmosphere causes a CO2 increase in the ocean.
Henry's Law is temperature dependent and warmer liquids hold less gas than colder liquids. This temperature dependence explains why a warm soda is much more likely to fizz out of the bottle than a cold soda and has large implications for regional differences in ocean acidification.
There is a huge range in temperature (and consequently CO2 partial pressure) throughout the ocean. For example, deep ocean water that has recently been in contact with the atmosphere contains more CO2 than surface water for two reasons. Firstly, it is colder so it can hold more CO2. Secondly, falling organic matter – fish poo and the like – gets eaten by bacteria as it falls to the bottom - releasing CO2 by respiration. Exchange of cold, CO2-rich deep ocean water and surface water happens through upwelling and downwelling. This can result in some ocean regions being sources of CO2 as deep water comes to the surface, warms up and degasses. Other regions are sinks of CO2 as warm water with low CO2 cools down, sucks up extra CO2 and sinks.
How can we be sure that ocean acidification is caused by CO2 in the atmosphere? In the next post, we'll discuss how we know that the extra CO2 in the atmosphere is not coming from a natural warming of the ocean.
*This was to be the 8th post in the series but we intercalated one and lost the original title for this post: "Henry the 8th I am".
Written by Doug Mackie, Christina McGraw , and Keith Hunter . This post is number 9 in a series about ocean acidification. Other posts: Introduction , 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.































 Arguments
Arguments

























 0
0  0
0 second summary post
second summary post







Comments