





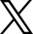




















Latest Posts
Archives
|
 |

Stratospheric Cooling and Tropospheric Warming
Posted on 1 December 2010 by Bob GuercioThis post has been revised at Stratospheric Cooling and Tropospheric Warming - Revised Increased levels of carbon dioxide (CO2) in the atmosphere have resulted in the warming of the troposphere and cooling of the stratosphere. This paper will explain the mechanism involved by considering a model of a fictitious planet with an atmosphere consisting of carbon dioxide and an inert gas such as nitrogen at pressures equivalent to those on earth. This atmosphere will have a troposphere and a stratosphere with the tropopause at 10 km. The initial concentration of carbon dioxide will be 100 parts per million (ppm) and will be increased instantaneously to 1000 ppm and the solar insolation will be 385.906 watts/meter2. Figure 1 is the IR spectrum from a planet with no atmosphere and Figures 2 and 3 represent the same planet with levels of CO2 at 100 ppm and 1000 ppm respectively. These graphs were generated from a model simulator at the website of Dr. David Archer, a professor in the Department of the Geophysical Sciences at the University of Chicago and edited to contain only the curves of interest to this discussion. The parameters were chosen in order to generate diagrams that enable the reader to more easily understand the mechanism discussed herein. Prior to discussing the fictitious model, consider a planet with no atmosphere. In this situation light from the sun that is absorbed by the surface is reemitted from the surface. Figure 1 is the IR spectrum of this radiation which is known as Blackbody radiation. 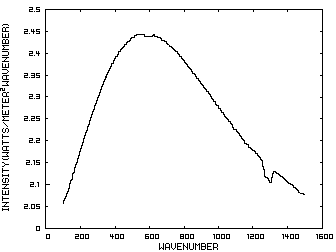
Figure 1. IR Spectrum - No Atmosphere Consider now Figure 2 which shows the Infrared (IR) radiation spectrum looking down at the planet from an altitude of 10 km with a CO2 concentration of 100 ppm and Figure 3 which shows the IR spectrum with a CO2 concentration of 1000 ppm. Both figures represent the steady state and approximately follow the intensity curve for the blackbody of Figure 1 except for the missing band of energy centered at 667 cm-1. This band is called the absorption band and is so named because it represents the IR energy that is absorbed by CO2. IR radiation of all other wavenumbers do not react with CO2 and thus the IR intensity at these wavenumbers is the same as that of the ground. These wavenumbers represent the atmospheric window and is so named because the IR energy radiates through the atmosphere unaffected by the CO2. The absorption band and the atmospheric window is the key to stratospheric cooling. 
Figure 2. CO2 IR Spectrum - 100ppm Figure 3. CO2 IR Spectrum - 1000 ppm The absorption band in Figure 3 is wider than that of Figure 2 because more energy has been absorbed from the IR radiation by the troposphere at a CO2 concentration of 1000 ppm than at a concentration of 100 ppm. The energy that remains in the absorption band after the IR radiation has traveled through the troposphere is the only energy that is available to interact with the CO2 of the stratosphere. At a CO2 level of 100 ppm there is more energy available for this purpose than at a level of 1000 ppm, thus the stratosphere is cooler for the higher level of CO2 in the troposphere. Additionally, the troposphere has warmed because it has absorbed the energy that is no longer available to the stratosphere. One additional point should be noted. Notice that the IR radiation in the atmospheric window is slightly higher in Figure 3 than Figure 2. This is because the temperature of the troposphere has increased and in the steady state condition, the total amount of IR entering the stratosphere in both cases must be the same. That total amount of energy is the area under both of these curves. Thus, in Figure 2, there is more energy in the absorption band and less in the atmospheric window while in Figure 3, there is less energy in the absorption band and more in the atmospheric window.
In concluding, this paper has explained the mechanism which causes the troposphere to warm and the stratosphere to cool when the atmospheric levels of CO 2 increase.  0 0  0 0
Printable Version | Link to this page
Spaceman spiff and Sphaerica have hit the nail on the head.
Bob, you really do have to ask yourself why the tropopause is cooler than the stratosphere. Its a contradiction to this article. The measured LW spectrum often shown, are showing that in the co2 band, its escaping to space at around 220k, and given the fact that the tropopause is cooler than the stratosphere, we can safely assume its below this in the troposphere that the average LW escapes to space from co2. Some will be absorbed by co2 and O3, but thats not the predominant reason for the T profiles, or why elevating co2 should cause the stratosphere to cool.
 0 0  0 0 0 0  0 0mostly right. You miss two key facts. First, all GHGs emit as well as
absorb, and whether you will get warming or cooling in a region depends on
the ratio of the change in absorption and the change in emittence.
Second, the troposphere has many IR absorbers, the stratosphere only two
(CO2 and O3 - everything else is minor). So the impact of CO2 above the
tropopause is amplified.
 0 0  0 0 0 0  0 0 0 0  0 0 0 0  0 01 2 3 4 5 Next
You need to be logged in to post a comment. Login via the left margin or if you're new, register here.
|

|





The Consensus Project Website
THE ESCALATOR

(free to republish)
|

































 Arguments
Arguments























 0
0  0
0







Comments