How we know the greenhouse effect isn't saturated
Posted on 13 February 2014 by Glenn Tamblyn, jg
This is the Basic rebuttal to the myth 'CO2 effect is saturated'
The mistaken idea that the Greenhouse Effect is 'saturated', that adding more CO2 will have virtually no effect, is based on a simple misunderstanding of how the Greenhouse Effect works.
The myth goes something like this:
- CO2 absorbs nearly all the Infrared (heat) radiation leaving the Earth's surface that it can absorb. True!
- Therefore adding more CO2 won't absorb much more IR radiation at the surface. True!
- Therefore adding more CO2 can't cause more warming. FALSE!!!
Here's why; it ignores the very simplest arithmetic.
If the air is only absorbing heat from the surface then the air should just keep getting hotter and hotter. By now the Earth should be a cinder from all that absorbed heat. But not too surprisingly, it isn't! What are we missing?
The air doesn't just absorb heat, it also loses it as well! The atmosphere isn't just absorbing IR Radiation (heat) from the surface. It is also radiating IR Radiation (heat) to Space. If these two heat flows are in balance, the atmosphere doesn't warm or cool - it stays the same.
Lets think about a simple analogy:
What might we do to increase the water level in the tank?
We might increase the speed of the pump that is adding water to the tank. That would raise the water level. But if the pump is already running at nearly its top speed, I can't add water any faster. That would fit the 'It's Saturated' claim: the pump can't run much faster just as the atmosphere can't absorb the Sun's heat any faster
But what if we restricted the outlet, so that it was harder for water to get out of the tank? The same amount of water is flowing in but less is flowing out. So the water level in the tank will rise. We can change the water level in our tank without changing how much water is flowing in, by changing how much water is flowing out.
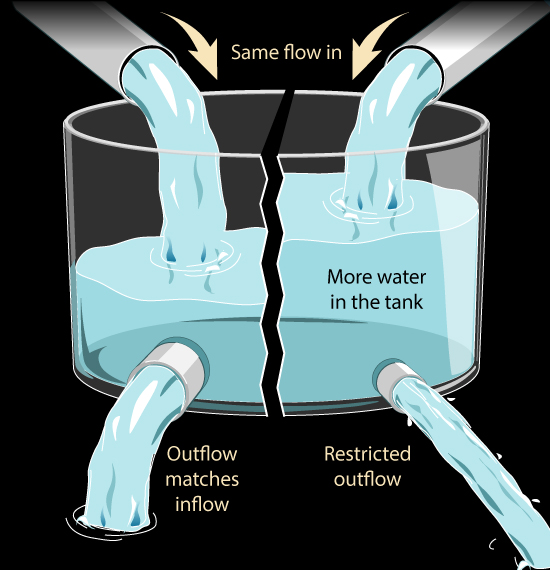
Similarly we can change how much heat there is in the atmosphere by restricting how much heat leaves the atmosphere rather than by increasing how much is being absorbed by the atmosphere.
This is how the Greenhouse Effect works. The Greenhouse gases such as carbon dioxide and water vapour absorb most of the heat radiation leaving the Earth's surface. Then their concentration determines how much heat escapes from the top of the atmosphere to space. It is the change in what happens at the top of the atmosphere that matters, not what happens down here near the surface.
So how does changing the concentration of a Greenhouse gas change how much heat escapes from the upper atmosphere? As we climb higher in the atmosphere the air gets thinner. There is less of all gases, including the greenhouse gases. Eventually the air becomes thin enough that any heat radiated by the air can escape all the way to Space. How much heat escapes to space from this altitude then depends on how cold the air is at that height. The colder the air, the less heat it radiates.
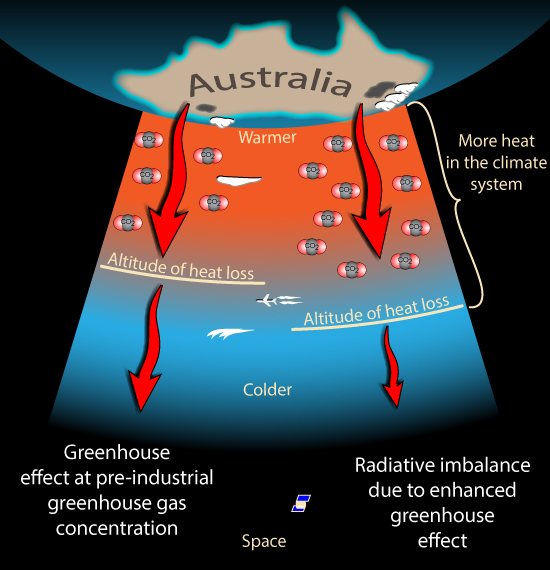
(OK, I'm Australian so this image appeals to me)
So if we add more greenhouse gases the air needs to be thinner before heat radiation is able to escape to space. So this can only happen higher in the atmosphere. Where it is colder. So the amount of heat escaping is reduced.
By adding greenhouse gases, we force the radiation to space to come from higher, colder air, reducing the flow of radiation to space. And there is still a lot of scope for more greenhouse gases to push 'the action' higher and higher, into colder and colder air, restricting the rate of radiation to space even further.
The Greenhouse Effect isn't even remotely Saturated. Myth Busted!































 Arguments
Arguments






























atmosphere isn't full of CO2 and other greenhouse gases, thus the greenhouse effect isn't saturated.
I understand and agree with this post. But I have a problem with the second bullet in the introduction. I always thought that there are a lot more emitters
of IR photons (and thus more IR photons) than there are greenhouse absorbers since the rock/ground molecules are packed to higher densities than the amospheric CO2 gas molecules. So even if all the CO2 molecules present at a given time were saturated (ie excited to higher energies via IR photon absorption), the new molecules entering the atmosphere via antropogenic means would capture more of the available IR photons. This would further reduce the net IR output at the top of the atmosphere
and further heat the atmosphere near the ground. Here the atmosphere is never in balance (ie in thermal equilibrium) and this cause the heat to accumulate near the ground. Another corrolary is that the atmosphere is never completely saturated: there are always more (new) CO2 molecules being introduced to capture the yet untapped IR photons available, and this further heats the atmosphere.
Is this picture correct?
potvinj
If you contrast the density of a solid surface with the density of the air there is a difference of the order of 1000's of times. Add in the proportion of GH gases in the atmosphere and the difference becomes of the order of 10's of 1,000,000's.
However IR radiation from a solid surface only originates from a very thin layer right at the surface, no more than microns thick. In contrast the atmosphere is kilometers thick. So this compensates so that there are ample GH molecules to potentially intercept IR photons. We need to contrast the number of molecules in a microns thick layer of a dense material with the number in a 1000's of meters thick layer of a diffuse material.
A different way of looking at this is to take how much energy is radiated from a solid surface at a given temperature based on Planck's Law and work out how many IR photons are radiated per second by dividing that by the average energy of the individual photons.
When we do that we get a result that says there is roughly the same number of CO2 molecules in a few cubic meters of air as there are photons being radiated from a square meter of a solid surface every second. When we then remember that a GH molecule is continuously transferring energy to all the other molecules around it through collisions - each molecule in the lower atmosphere collides with other molecules billions of times per second - and thus the GH molecule can potentially engage in an abroption event millions of times a second or more.
So there are actually millions of CO2 molecules available to potentially interact with each IR photon emitted from the surface in the first meter of that photons travel. If it were based just on the probability of them meeting all the IR radiation would be absorbed within the first meter of the atmosphere.
However just because a photon and a molecule encounter each other does not automatically mean that absorption occurs. There is actually only a modest probability of an absorption event occurring. It is this that extends the distance that it takes for most IR photons to be absorbed out to typically 100's of meters.
potvinj @2, there are over 830 x 10^12 Kg of CO2 in the Earth's atmosphere (equivalent to 390 ppmv). The density of dry ice, ie, the precipitated form of CO2 is, 1,600 Kg per cubic meter. The area of the Earth is 510 x 10^12 meters squared. Consequently, if the atmospheric CO2 weref precipitated out into an even layer across the Earth's, there surface would be 1.6 Kg per square meter, or a layer 1 millimeter thick across the Earth's surface.
The question then becomes, can a layer a millimeter thick be effectively opaque to a given frequency. Could we, for example, coat a sheet of glass with a layer of paint less than a millimeter thick such that it would block out effectively all light?
Put in these terms, it is apparent that it is physically possible, and measurements have shown it is actually the case, that the atmosphere is effectively opaque to IR radiation in a limited bandwidth. This does not stop radiation of IR in that bandwidth to space, because the CO2, including CO2 above 80% of the CO2 in the atmosphere itself radiates in that bandwidth, with the radiation from the upper levels of CO2 reaching space.
The reason for the qualifier in the second bullet point is that CO2 absorbes less and less well as frequency moves from the center of the bandwidth of maximum absorption. The result is that IR radiation in those frequencies that would have previously escaped have an increasing chance of being absorbed with increased CO2. It should be noted that in the very center of the CO2 absorption band, the majority of emissions to space are from CO2 in the stratosphere, which is warmer than the upper troposphere, and warms with increased altitude. Consequently the mechanism described in the OP does not work (indeed, has the opposite effect) in the center of the absorption band. It does, however, work in the wings, so that the net effect is as described.
jyhh @1, if you were being witty and making a pun, forgive my obtuseness.
If not, your argument is a non-sequitor. If CO2 absorbed at all IR wavelengths at its maximum strength, it would be saturated, even at current CO2 concentrations. That is because at maximum strength its effective altitude of emission is in the low stratosphere, which warms with altitude. Therefore increased CO2 would increase the effective altitude of emission, and hence increase the IR radiation to space.
Of course, my argument assumes hypothetically that the temperature structure of the atmosphere would remains the same in such a case. It is far from clear that that would be the case with 100% IR absorbing CO2. The hypothetical, however, is sufficient to show your argument fails logically. That is, you may be able to infer that which you wish to, but you would need far more premises to do so than you actually give.
As it happens, even with a 100% CO2 atmosphere, so long as the temperature at the effective altitude of radiation to space fell with increasing altitude, increased CO2 would result in less IR radiation to space at a given surface temperature, thereby requiring an increase in surface temperature to maintain the energy balance. That is, even 100% CO2 atmospheres need not be, and typically would not be, saturated.
yes, Tom Curtis, let's not get semantic on the words 'greenhouse gas' or 'greenhouse effect'. But I bet my explanation is better for 8-year-olds than for intentionally obtuse deniers. I also think there could be some gases that would rise the surface temperature of Venus still, so the greenhouse effect isn't saturated there either. I haven't come up with one but then I haven't particularly wanted to find out. Local concetrations of gases vary of course, but they stay the same and do not change their properties. 100% IR-absorbing material sounds like something scary from a scifi-novel, but that isn't what you said, the IR-photons can wiggle their way out of the planet between the molecules even in a 100% CO2 atmosphere.
Actually jyyh, this myth only relates to the idea that adding more CO2 can't cause much more warming.
What you are referring obliquely through you reference to the magnifying glass is the 'it's the Sun' myth
As someone without a backround in science I found the above explanation confusing. For example heat energy radiated back into space would need to pass through the high altitude cold air whether it came from near the surface or higher in the atmosphere. No explanation is given as to whether CO2 as it accumulates higher in the atmosphere is more effective at trapping heat because there is perhaps less water vapour. Which ever way I look at it I fear climate change sceptics have little to fear from this latest effort at myth busting.
This remains one of the trickiest things to explain to us laypeople. Acronyms and nomenclature don't make it any easier.
Eg, the reference to 'TOA' - top of atmosphere. Unless someone is very conversant with the dynamics, then they wouldn't know if you were talking about the top of the troposphere or some place higher. Mention stratospheric cooling and one assumes that TOA refers to the upper limit of the troposphere. But mention some unnamed point where "heat is finally radiated out to space," and many people will inlcude the stratopshere, mesosphere, ionosphere - the whole of the atmosphere.
I know that the altitude of the troposphere is increasing and still have difficulty understanding which part of the atmosphere is the thinnest part where radiation finally escapes to space, and what exactly is meant by 'TOA'.
One explanation describes the atmosphere in even more discrete layers. Is the saturated layer radiating to a higher one that is less saturated (the blanket explanation)? Wouldn't more CO2 narrow the window at the highest altitude, where there should be no saturation? Or is saturation in the 15 micron band complete at all alittudes, leaving only the 'wings' of the spectral band unsaturated?
I've read the realclimate articles and dozens of others on saturation and explanations vary, seemingly contradictorily on some points. I suppose this might be because at the molecular level normal physical analogies don't quite capture the reality.
Still questing - any further clarity will be appreciated.
Richard McGuire - yes, CO2 is more effective in the upper atmosphere because the air is colder and less humid, however that is a more advanced topic than is intended to be covered in this particular article.
The argument that CO2 is saturated was first raised by Knut Angstrom in 1900, and was refuted in the forties and fifties by the work of Guy Callendar and Gilbert Plass, so the skeptic myth was busted over fifty years ago. The skeptics are rather behind the times to begin with on this one! This argument is a bit of a touchstone, a bit like the argument that the rise in atmospheric CO2 is natural; it is an indication that the "skeptic" simply can't be bothered to look into the science and find this myth was busted long ago.
There is a slightly more detailed article at RealCLimate that is well worth reading (in addition to the many others)
The only way a myth can be debunked is if what is put up on science blogs such as this can be explained , debated and understood in the wider community. The saturation argument is a popular with climate change contrarians. I think the Real Climate "Saturated Gassy Argument" referred to @9 is well worth a read, though even there a better explanation is required as to why the cold layers in the upper atmosphere do not also impede the escape of heat energy radiated from the surface.
Thanks Glenn & jg for the simplest possible explanation of the essence of GHE. I would not imagine such explanation be possible as the more i'm learning about GHE the less easy it becomes, as the grasping of many aspects of physics is required.
Your last three paragraphs do capture the essence of GHE from the planetary energy balance perspective. But on top of that, the average person or denier might ask: what does such effect has to do with supposed increase of surface temperature everyone is fearfully talking about? The answer is: as the 'action' moves higher, the temperature profile of the atmosphere must adjusts according to the temperature lapse rate. You have to refresh your knowledge about lapse rate (dry and wet) in order to understand why such adjustment is happening. Just as you have to recall the adiabatic processes in order to know why the air pressure and temperature decrease with altitude. Also you have to be aware why permanent gases like CO2 are "well mixed" while H2O precipitates at tropopause. Those basic processes are not explained in this article because no one seems to be denying them (although few years back, someone in US Congress was speculating that "excess CO2 will stay near ground because it's heavier than rest of gases", so even such basic high school topic sometimes requires debunking).
I don't want to discuss details of planetary response to GHG forcing: that's a separate topic. I just wanted to show a nice animation from RC, that captures the situation like 1000 words:
From there, you can now clearly see that a property of constant lapse rate - the slope of the blue line - in the atmosphere (more or less true in the first approximation) ensures that the increased temperature near the top of troposphere is transfered all the way back to the ground, resulting in GW as we the ground creatures, experience.
Richard McGuire, the cold layers in the upper atmosphere stop impeding the escape of heat energy radiated from the surface simply because the density of CO2* falls below the threshold that insures that a photon will more likely be absorbed rather than continue to space unimpeded. Since that altitude is colder than the surface, less energy is radiated to space than is emitted by the surface, so the entire atmosphere below that altitude warms until outgoing energy matches incoming energy.
Adding more CO2 makes little direct difference at the surface, but it raises the altitude where CO2 radiates to space, and since that higher altitude is colder, the entire atmosphere below that altitude will warm still more until outgoing energy once again matches incoming energy.
*H2O is simply not a factor at the altitude where CO2 radiates to space as H2O is almost non-existant, having dropped below the concentration of CO2 between 6 and 8 km up, and fallen to only 3-4 ppm at the tropopause. However, as the atmosphere warms it will hold more H2O, thus raising the altitude where H2O radiates directly to space in wavelengths not absorbed by CO2, which will cause still more warming of the atmosphere below that altitude. This is known as the water vapour feedback.
Chriskoz, that graphic is a close representation of how I imagine the increase in surface temps from a steady lapse rate but increasing height of troposphere from GHG warming. I believe there is a small caveat to add - the lapse rate does not remain quite constant, providing a small negative feedback, but not nearly enough to counteract the impact on surface temps from the elevated tropopause (this from meory - someone please correct if I am wrong).
I am always reminded that saying enough to be clear without reneging on the whole truth is often difficult to balance. Like a documentary maker who has a limited frame and time in which to capture the essence of the subject as well as possible.
@ 15 Jim Eager your explanation was helpful.
Thanks for this article: I will be linking to it often, in combating denialospheroids at various blogs.
I'm glad you found it clear enough to be helpful, Richard. The animated diagram that chriskoz posted nicely illustrates the increase in effective radiating altitude that I described. The difference in temperature as the radiating altitude increases is crucial to the greenhouse effect, and it is a concept not explained in most general descriptions. The effective radiating altitude doesn't have to increase by very much, either. At the emission altitude of CO2 the dry lapse rate (remember, there is almost no H2O at that altitude) is around 1C per 100 meters.
barry@15,
You're correct: the negative lapse rate feedback parameter overall is about -0.8W/m2K, an absolute value smaller than e.g. the H2O feedback (parameter +1.8W/M2K. See IPCC AR4 and Soden 2005.
However, as you might guess, the LR feedback is not uniform nor positive everywhere:
Near the poles, LR feedback happens to be slightly positive (last picture) while negative (second last picture) in the tropics. Overall the tropics influence is larger, hence its average value -0.8W/m2K.
Thank you Glenn Tamblyn (#3) and Tom Curtis (#4) for showing the error of my thinking (#2). But - still - the 2nd bullet in the intro still rings false to me. Surely, one needs a mechanism to warm up the lower portion of the atmosphere in order to push upwards the height of the high altitude radiative layer, as eloquently sketched in the 2nd diagram of the OP. What is it?
Reading the 2nd sentence of Tom's 3rd paragraph (#4; about the absorption "wings"), it would follow that the lower atmosphere warms up further (in comparison to the pre-industrial regime) because adding CO2 increases the absortion likelyhood over a wider bandwith by adding more absorbers. In other words, those extra absorbers would be more likely to tap photons that used to pass through the lower atmosphere (here are my "untapped photons" again). So it would follow that the deniers' claim that "...adding more CO2 won't absorb much more IR at the surface..." is FALSE. The (lower) atmosphere is not saturated, currently.
Note: Some readers may be thinking that the lower atmosphere heats up more simply b/c less radiation leaks out to space if that radiative layer indeed ends up at a higher altitude. True enough. But what causes it to go up in the first place - say during the times of the beginning of the industrial revolution? To me, it would seem that the reduction of radiative leakage at high altitude acts as a GW positive feedback rather than a direct cause.
potvinj @20, briefly, convection in the troposphere is so strong that it dominates radiation as a method of energy transfer in the atmosphere. The consequence is that within the troposphere the relative temperature at different levels is tightly constrained by convection. If you increase CO2 concentrations, that reduces the escape of heat to space from higher levels of the troposphere. The resulting warm high altitude air slows the rate of convection, which thereby slows the rate of energy rising from near the surface to the upper troposphere. That warms the surface. After the surface has warmed sufficiently, convection returns to its prior levels. This process is quite fast, taking a few hours or so. It is also difficult to detect against the very varied background of changes in hourly rates of convection due to weather and day night cycles.
like to show this videotaped response to Dr. Fred Singer's (hired denialist) question on the subject dr. Roy Spencer. His basic response is, "pressure broadening" and "we can measure it increasing so it is not saturated"
www.youtube.com/watch?v=potLQR7-_Tg&t=1h1m
Others here have already explained how the greenhouse effect shifts the Earth’s heat loss to space from the relatively warm and strongly radiating surface up to a colder and therefore less radiating layer in the atmosphere. That altitude is about 5 km on average, but varies a lot depending on the frequency because the atmosphere is virtually 100% opaque in some parts of the infrared spectrum and almost completely transparent in others. The image below (created with the MODTRAN radiation model) shows the source of the radiation escaping to space in different parts of the IR spectrum when CO2 concentration is 400 ppm and the surface temp is 300 K.
Note the thin, upward spike (4) at the centre of the CO2 band. That’s the signature of the warmer stratosphere compared to the very cold, upper troposphere. As the CO2 concentration continues to rise, most of the extra absorption will happen in the “wings” (2) of the big CO2 “bite”, not at the bottom (3) where the radiation already comes from the coldest part of the atmosphere.
potvinj
to comment further on Tom's point. We need to differentiate between the mechanisms that can add heat to the atmosphere, and those that can then move heat around the atmosphere once it is there.
From Trenberth, Fasullo & Kiehl 2009 the estimated energy flows into the atmosphere are:
So radiation dominates the the sources of heat transfered into the atmosphere. However once absorbed by the atmosphere radiation is then a very poor method of moving heat around within the atmosphere. Every time some radiation occurs it is quickly absorbed and then reradiated in a random direction, making it very hard for radiation to transfer heat in one direction quickly - this is the opposite of what happens when radiation isn't being absorbed by the medium. A similar situation occurs inside the Sun where it can take 100's of 1000's of years for heat to percolate out from the core via radiative transfer.
In contrast air movements rapidly move heat up and down and from one loaction to another. So air movement will quickly propagate a change in one location to bring other parts of the atmosphere into balance with it.
To provide further explanation to potvinj@20 question, the analogy to the water heating in a kettle can be considered. The water is heated from the bottom only but being poor heat conductor, it would not be possible to heat its entire volume evenly by conduction only. But convection processes cause bottom parcel rising and top parcels falling creating mixing turbulences and assuring that heat distributes evenly.
The same convection mechanisms happen in gases like air that's also heated from the bottom by the ground absorbing the incoming sun's radiation: the experimental proof is the weather and the rising currents used by gliders/birds as a lift-off. The only difference is that water in a kettle ends up with the same temperature in its entire volume while atmosphere's air temperature equilibrium is defined by the lapse rate as I've shown @13
The absorption band of CO2 centred at 667 cm-1 (15 microns) grows wider as higher concentrations of CO2 make the atmosphere opaque at frequencies where it previously was quite transparent. This moves the altitude of heat loss in the “wings” of the CO2 band from the low or middle troposphere up to the cold tropopause while the central part of the band moves to the warmer stratosphere.
These spectra (made with MODTRAN) haven’t taken into account the rising surface temperature, the higher tropopause and the cooling stratosphere caused by more CO2. The latter will partly offset the growing spike in the middle of the absorption band and thus restrict the heat loss to space even more than shown here.
The impact from CO2 is very far from saturated!
Glenn explains it pretty well here, in terms that anyone familiar with the subject can understand. But a few readers are still having trouble coming to grips with this, so I refer you to a masterful science communicator, Spencer Weart:
http://www.realclimate.org/index.php/archives/2007/06/a-saturated-gassy-argument/
The takeaway paragraph from that article is:
"What happens if we add more carbon dioxide? In the layers so high and thin that much of the heat radiation from lower down slips through, adding more greenhouse gas molecules means the layer will absorb more of the rays. So the place from which most of the heat energy finally leaves the Earth will shift to higher layers. Those are colder layers, so they do not radiate heat as well. The planet as a whole is now taking in more energy than it radiates (which is in fact our current situation). As the higher levels radiate some of the excess downwards, all the lower levels down to the surface warm up. The imbalance must continue until the high levels get hot enough to radiate as much energy back out as the planet is receiving."
This is for everyone who has answered (mostly) in response to potvinj. Two comments and a question.
1) If everyone is in agreement that convection is the first-order mechanism for transferring energy from the surface to the upper level of the troposphere, from where it radiates to space, could you reference or produce a graphic that illustrates this? The images here and elsewhere seem to support the radiation-from-the-surface-up model.
2) For people in the colder parts of the world, I think there's quite a simple analogy that would be very accessible. Most of us are aware that the warm air in their houses rises to the ceiling, and that better attic insulation is one of the easiest ways to increase the temperature. We also have been exposed to the fact that different materials have different values of insulation.
I would explain the CO2 effect in these terms: A higher percent of CO2 in the upper layers of the troposphere is like replacing vermiculite (found in much older homes) with fiberglas; the R-value is greater, so your house is warmer for the same heat input. (I speak, grateful today, from the experience of having done it.)
3. Here I must admit my own confusion. Following the analogy, if I have better insulation, I need a thinner layer of it to achieve the same effect. So, as I add CO2, the thickness of a layer of atmosphere required to achieve opacity should decrease. Perhaps someone could, again, offer a diagram that give some kind of perspective or scale on those upper levels of the troposphere that are subject to the effect (not already opaque.) I think I understand the basic physics, but the expression "moving up" applied to either radiating or translucent layers is causing me trouble.
(Note: By opaque I mean absorbing and re-radiating all photons available.)
Thanks.
mgardner,
on your 1): see the post by Glenn @24: convection is contributing to heat transport from the surface to the atmosphere, but not the majority; that is still IR radiation
on your 3): Yes, the insulation analogy works, aka more CO2 in a layer lets you make that layer thinner for the same opacity (of IR from the layer below reaching the layer above). More CO2 means such opagueness at a certain wavelength is achieved earlier, but "moving up" is not meant literally except in the sense that the whole tropospheric column has to warm, and thus any layer of a given tropospheric temperature will, on average, be at a higher elevation under an increased greenhouse gas concentration
Ultra Basic Rebuttal : Venus.
Interesting factoid: because Venus has such a high albedo, both Earth and Venus have about the same incoming solar radiation. But Venus is several hundred degrees hotter.
@29 gws
Thanks for your response, but I think you are misreading Glenn and the others. See Tom @21.
I also think you are missing the point on the altitude question.
I'm hoping those guys can turn their attention to this when they are done pummeling Russ. I would have lost patience long ago.
gws wrote: "on your 1): see the post by Glenn @24: convection is contributing to heat transport from the surface to the atmosphere, but not the majority; that is still IR radiation"
I suspect you have misunderstood Glenn's post, IR radiation transfers heat from the surface to the layer of air immediately above the surface, where it is absorbed. Convection then transfers this heat to the bulk of the trophopshere.
"but "moving up" is not meant literally"
No, the height at which IR is radiated into space really does move up as more CO2 is added to the atmosphere. Think of the thickness of the layer required to obtain a certain level of opacity at the top of the atmosphere. As CO2 is added, this thickness decreases and so the bottom of the layer becomes higher. The bottom of this layer is then cooler (due to the lapse rate) and so emits less IR, creating the energy imbalance.
@30:
Actually, Venus gets even less solar energy than the Earth does!
Most sources I’ve found state that Venus’ albedo is about 75%, while the Earth’s albedo is 30%.
Taking into account the 91% higher insolation because of the shorter distance to the sun, the net result is that Venus receives about 32% less solar energy per unit of area than we do.
If Venus had no greenhouse effect at all, its average temperature should be about -40oC, or more than 500oC colder than it really is! (Assuming the solar energy was evenly distributed. The temperature variations caused by the day-night cycle and different latitudes would lower the average temperature even more)
In short, the Earth's energy budget is determined by the balance of incoming and outbound radiation, so rather than thinking about what happens to the IR radiation that is emitted by the surface, you need to think of where the IR that actually does escape the Earth comes from. The key is to think about the IR radiation that isn't absorbed by CO2, rather than the IR that is.
@32 Dikran Marsupial
"As CO2 is added, this thickness decreases and so the bottom of the layer becomes higher."
Perhaps I really do need to have a picture drawn to have this make sense. Let's say there's an altitude h which represents the top of the top opaque layer O. Above it there is a translucent layer, Lt1. For the bottom of Lt1 to move up, the top of O must also move up, but if it is already saturated, then adding CO2 should have no effect on O; its thickness remains constant. It seems more logical that the top of Lt1 moves down in altitude.
What effect am I missing?
mgardner - The opacity to IR in absorbance bands is set by the total amount of GHGs. As the concentration of GHGs increases, the atmospheric pressure where that total amount is small enough for IR to reach space decreases, meaning it occurs at a higher (and colder) altitude.
In short, as GHG concentrations increase, the bottom of the 'translucent' layer becomes IR IR absorbing, and the radiation altitude increases - the thickness of atmosphere wherein IR is captured does indeed become thicker.
mgardner, the top of the opaque layer is determined by the bottom of the translucent layer, rather than the translucent layer being determined by the top of th opaque layer.
Imagine we needed a layer 1km thick to be completely opaque. That doesn't mean that the atmosphere above 1km is traslucent, the next 1km layer will also be opaque. However the energy from IR emitted from the surface that is absorbed by the first opaque layer is still transfered to the one above it by conduction, convection and radiation. There could be several such saturated opaque layers from which IR cannot pass through from thelayer below, even though some of the heat energy does pass through them.
IIRC the lower atmosphere isn't completely opaque to IR, so it probably isn't best to think too strongly about it in that way, instead there is a band in the atmosphere from which the bulk of IR is radiated. Some may come direct from the surface, but not much as it is absorbed in the trophosphere, most will be emitted from the air higher up in the atmosphere, which is cold. The more CO2 you add, the higher in the atmosphere it needs to be radiated from in order not to be absorbed before it escapes. I suspect there is a probability distribution that shows the density of the height in the atmosphere from which IR photons escape; the mean increases with increasing CO2.
KR and DM
Thanks; I do understand the principles as described. I guess my problem is with this language of 'layers'. It seems that even you guys aren't quite in agreement on how to characterize what's happening when you try to employ it.
The analogy I used @28 fails in a strict sense because it is restricted to radiative transport; there would have to be some other metaphor for mass effects (convection), to account for the gradient or thermocline through this upper zone of the troposphere where the action is. (I'm still hoping someone can refer me to a source that gives a higher-resolution picture of that area.)
But maybe the real source of confusion is that opacity to 'original photons' makes us think of 'layers' when there are no layers with respect to radiative transport? Or do I have that wrong?
I don't think I am saying anything substantially different from KR, I was just giving the caveat that there probably isn't a clear boundary between the "opaque layer" and the "translucent layer", just that the probability of an IR photon escaping into space depends on the height at which it is emitted, and that as more CO2 is added, the higher the mean altitude will be. The boundary between the layers is equivalent to the height at which the p.d.f. starts rising quickly, but it isn't a distinct boundary. If it makes more sense to think of a p.d.f. than in terms of layers, then think of a p.d.f.
Layers are often useful in explaining concepts about the atmosphere, but they layers don't always correspond to physically distinct layers in the atmosphere (like the trophosphere and stratosphere). Like all analogies/models, there comes a point in the discussion where you need to move onto a lower level of abstraction. IIRC layered models are often used in practice (atmosphere and ocean) as it is easier to model a continuously varying atmosphere as a number of thin homogenous layers.
mgardner - Discretization of a continuous system is a useful approach for analysis, but one must always keep in mind that it is an approximation of a continuous reality.
There are certainly qualitative dividing lines that can be drawn - the top of the troposphere where convection ends and the still air of the stratosphere begins, for example. But the boundary for IR emission for each greenhouse gas (different for each) is a probabilistic one due to concentration based chances of interception - with < 50% of upward IR from slightly below that altitude escaping to space along with > 50% of upward IR from slightly above.
DM @39 or anyone who can contribute:
I'll give it one more try; perhaps I'm not communicating very well, or I'm completely wrong in my understanding.
1) The probability of a photon being emitted depends on the temperature, which depends on the altitude.
2) The probability of that photon escaping absorption and re-radiation (meaning attentuation statistically) is dependent on the altitude and the density of CO2 above it.
So, there are some photons that escape completely, and there is some attenuation of the total transport from that level through absorption and re-radiation in the atmosphere above the level of emission. What I'm trying to find is the quantitative relationship between those effects. I don't require great certainty, just some relative values.
If this is something we don't know, that's fine, but does anyone know if we know or don't know? (No, I'm not Rumsfeld in disguise.)
KR@40
Please note: I'm the one who wasn't comfortable with the whole "layers" thing in the first place. Maybe you could take a look at my @41; I think that's the best I can do at posing the question.
mgardner & others:
Let’s try another approach and see if it helps:
1. The Earth receives 240 watts of solar energy per square metre on average when its spherical shape and albedo is taken into account.
2. To maintain a stable temperature, each square metre has to lose 240 watts back to space. This heat loss can only be in the form of infrared radiation because convection (dominant within the troposphere) and conduction is impossible in a vacuum.
3. The Earth’s average surface temperature is about 288 K (15oC), which corresponds to an IR emission of 390 watt/m2. That’s 150 watt/m2 more than needed to balance the energy received from the sun.
4. In order to avoid a dramatic cooling, the Earth’s heat loss to space has to occur from a level in the atmosphere where the emission equals 240 watt/m2. By using the Stefan-Boltzmann law (j* = T4 x 5.67x10-8) one find that this emission intensity corresponds to a temperature of 255 K (-18oC).
5. This means that the average altitude of heat loss to space is (288-255)/6.5 ≈ 5 kilometres above the sea level. Note that this altitude depends very strongly on the frequency of the emission. Some frequencies are hardly absorbed at all and therefore able to escape directly to space from the surface (see image in my post @23), while others can’t escape before they reach the lower or middle stratosphere because CO2 absorbs so strongly even in very low concentrations.
6. More CO2 and other greenhouse gases make the atmosphere at the altitude of heat loss more opaque to infrared, so the IR emissions from this altitude can’t escape to space so easily any more. As a result, the altitude of heat loss is pushed upwards to a thinner part of the atmosphere with fewer CO2 molecules above.
7. This new altitude of heat loss is colder and emits less heat to space, meaning that the Earth as a whole loses less heat to space than it receives from the sun.
8. As a result, the Earth has to warm until its heat loss to space increases enough to restore the balance between incoming and outgoing energy.
Did this help, or did I just create more confusion?
mgardner - The interception probabilities are due to the total GHGs above the emission point and atmospheric temperatures, which are entirely quantitative but not reducible to a simple expression. That's why tools such as MODTRAN are used, running line-by-line computations with discretization over many layers.
HK @43
A noble effort, but I already understand all this, and it doesn't answer my question or my concern about communicating it to others, which I thought was a major purpose of the blog. KR@44 is saying that it is possible to answer my question, but apparently I have to acquire and become proficient with MODTRANS, run my numbers, and whip up the graphics on my own time.
Here's an example of what I'm looking for:
Total
A nice image and explanation, I think. If I were explaining this to someone, I would use it, and I think most sincere 'students' would get it. But if someone then asked me "what about that saturation thing I've heard about", I would like to be able to zoom in on the zone at the top of the troposphere, and show a similar diagram with some approximate values.
I could elaborate, but I'm not sure if there is any point if there's no source of information.
HK et al
I did some more searching and came up with this:
If I understand correctly, we take something like 5km to be the altitude above which attenuation of IR by CO2 is negligible--"where energy radiates freely to space".
Now, without telling me about how it all varies with season and latitude, and all of the complications involved in doing the calculations, can I find out about what that altitude would be if we double the CO2 concentration? Is it 100m higher, or 1km higher, or 4 km or what? I can eyeball that the temp changes between 5-10C in 1 km.
I would also like to know, again, order of magnitude, what the thickness of an 'opaque' 'layer' just below that original 5 km altitude would be. And what the attenuation would be for IR radiation in some CO2 band through that 'layer'.
This is what I'd like to see represented on a graphic. I'm beginning to realize that there may not be one anywhere with that kind of resolution, and perhaps I will set up a chalkboard, draw some diagrams, take a picture, and post it here to have you guys check it out.
mgardner - The "...thickness of an 'opaque' 'layer' just below..." would be all the way from that altitude to the ground. And it's not, properly speaking, a single opaque to transparent transition, but rather increasing mean path length (10's of meters to kilometers) with decreasing GHG density, until ~50% or more of the IR photons in GHG frequencies are escaping to space.
Some additional resources for you: Trenberth et al 2009, Earths Global Energy Budget, figure 1:
[Source]
Also an excellent GHG Java simulation from PHET that shows absorption re-emission events varying with GHG concentration, as well as the ability to run some glass plate "layer" experiments.
mgardner wrote "And what the attenuation would be for IR radiation in some CO2 band through that 'layer'."
FWIW, I think you are missing the point, what matters is the temperature of the atmosphere from which outbound photons are not absorbed. It doesn't really matter how much IR is attenuated in the "opaque layer" as some of the energy of the absorbed photons will still be transmitted to the "translucent layer" via collisions, convection and re-radiation. As it happens, IIRC, the opaque layer isn't actually saturated either, but it would make no real difference if it was, or whether it had enough CO2 to be saturated ten times over. What matters is the energy of outbound IR that isn't absorbed on its way out into space.
You may find part 2 of the RealClimate blog post of some interest as well.
DM and KR @48 and @47
DM: "It doesn't really matter how much IR is attenuated in the "opaque layer" as some of the energy of the absorbed photons will still be transmitted to the "translucent layer" via collisions, convection and re-radiation."
What exactly do you think "attenuation" means, other than that?
KR: "The "...thickness of an 'opaque' 'layer' just below..." would be all the way from that altitude to the ground."
Well, no, it wouldn't be. There's some point below a given altitude h (e.g. 5km) at which photons emitted (in a given band) will not reach h. That's dependent on the density profile. And below that, there's another such point, and so on. What I don't know is how many such points exist before I reach the surface.
Look, there's nothing I'm asking that an actual expert would have trouble with-- it's the most basic of the physics of this subject.
So I'm leaving my question open for anyone who either has some ballpark numbers in their memory or can specifically refer me to where I can find them, or who can explain to me why they don't exist.
mgardner, the bit that is attenuated by absorption by CO2 will still be eventually transferred upwards by the mechanisms I mentioned, which is why the attenuation of the opaque layer is essentially irrelevant. Consider what happens to the energy absorbed by the opaque layer, if it is not to indefinitely increase in temperature, it must loose the energy either upwards to the translucent layer, or downwards to the surface (back-radiation).
The Earths energy budget is determined by the difference in incoming and outgoing radation. How many times the energy is transferred within the atmosphere before it is finally radiated out is irrelevant to the Earth's energy budget.