Joseph E. Postma and the Greenhouse Effect
Posted on 17 August 2011 by Chris Colose
Some recent attention has recently been going around the web concerning a new “paper” done by Joseph E. Postma (PDF here) which claims to “…physically negate the requirement for a postulation of a radiative atmospheric greenhouse effect.” It has been echoed particularly by some of the more crackpot web sources like climaterealists.com, and of course is spreading around various "skeptic" blogs.
The claims are of course extraordinary, along the lines of Gerlich and Tseuchner’s alleged falsification of the atmospheric greenhouse effect. As is often the case with these types of “skeptics,” the more extravagant the claim, the more obscure the publishing venue; in this case the host is Principia Scientific International, which according to the website “…was conceived after 22 international climate experts and authors joined forces to write the climate science bestseller, ‘Slaying the Sky Dragon: Death of the Greenhouse Gas Theory.’” Most rational people would stop here, but this is the Americanized age where we need to glorify everyone’s opinion and must provide rebuttals for everything, so here it goes:
I ask that the reader have the paper open in a new window so they can follow along with this article.
The Foundations
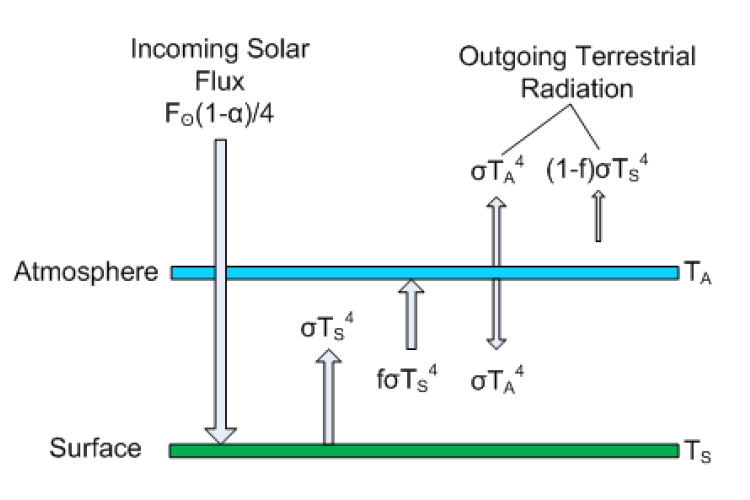
Most of Postma’s first 6 pages are actually correct. He describes the greenhouse effect through the so-called layer model, which is a simple way to break up the planet into a “surface” and an “atmosphere,” with outer space overlying the top layer. This model is described in many climate books such as Dennis Hartmann’s Global Physical Climatology, David Archer’s Understanding the Forecast, Marshall and Plumb’s Atmosphere, Ocean and Climate Dynamics, and radiation books like Grant Petty’s First Course in Atmospheric Radiation. I will say that I do not particularly like this model as a suitable introduction to the greenhouse effect. It is useful in many regards, but it fails to capture the physics of the greenhouse effect on account of making a good algebra lesson, and opens itself up to criticism on a number of grounds; that said, if you are going to criticize it, you need to do it right, but also be able to distinguish between understood physics and simple educational tools.
The atmosphere in Postma’s paper is just a single slab, so he has two layers (atmosphere+surface), but in general you can have many atmospheric layers of varying emissivity. He goes on to solve for the energy balance of each layer (see equations 11-14). RealClimate derived the same result in less than a page here.
Figure 1: Layer model is Postma's paper. Click to Enlarge
Postma actually doesn’t get the atmospheric radiative flux right. The emission is not σTa4, it is fσTa4, where f is the atmospheric emissivity/absorptivity (following his notation). The emissivity is a unitless factor between 0 and 1 descrbing how good of an absorber/emitter the object is relative to an ideal body. f = 1 describes a blackbody. By Kirchoff's law, the absorptivity of a layer must be equal to the emissivity (at the same wavelength), Both right hand sides of equations 11 and 12 are thus wrong, but it turns out that those errors cancel each other out and he gets equation 14 right. The factor of 2 in Equation 12 comes about because the atmosphere emits both up and down, although Postma clearly doesn't know how to derive this result formally, based on later statements he makes about this. Toward the end of page 14 he says this is invalid since the atmosphere radiates in 3-D, not just up and down. In fact, the quantity σT4 refers not only to the total power output of an object (the rate of energy emission), but it also refers to isotropic (equally intense in all directions) radiation. The result σT4 is obtained if one assumes that a plane radiates uniformly over a hemisphere (for example, the domed "half sphere" field of vision that a human can see when you stand outside," with the base of that half-sphere being the surface you sre standing on; the other hemisphere is invisible (see this image).
So far, it is simple textbook stuff with not much promise.
Geometry of the Global Energy Budget
Postma then goes on to describe fictitious “boundary conditions.” In particular, he seems to have serious objections to the averaging of the solar radiative flux over the Earth. In essence, he would prefer we had one sun delivering 1370 W/m2 of energy to the planet, with a day side and a night side, noon and twilight, etc. instead of the simple model where we average 1370/4=342.5 W/m2 over the planet (so that the whole Earth is receiving the appropriate "average" solar radiation). The number becomes ~240 W/m2 when you account for the planetary albedo (or reflectivity).
The factor of 4 is the ratio of the surface area to the cross section of the planet, and is the shadow cast by a spherical Earth. It is therefore a geometrical re-distribution factor; it remains “4” if all the starlight is distributed evenly over the sphere; it is “2” if the light is uniformly distributed over the starlit hemisphere alone; with no re-distribution, the denominator would be 1/cosine(zenith angle) for the local solar flux.
In simple textbook models, we like to prefer explanations that get a point across, and then build in complexity from there (see Smith 2008 for descriptions on a rotating Earth). Of course, students who use this model are probably educated to the point where they know that day and night exist, and certainly GCMs have a diurnal cycle. The radiative calculations are done explicitly by accounting for the temperature distribution and absorber amount that is encountered at each grid box. Postma is simply tackling a non-issue, just as how people criticize the term “greenhouse effect” for not working like a glass greenhouse. Postma objects to teaching this simple model because it is not real. All that is done, however, is to use a brilliant and sophisticated technique, taught only to the geniuses among us, called averaging! And of course, simple models are used in any classroom...it is how we learn.
But, in actuality, the globally averaged solar re-distribution approximation is not bad when we use it to describe the temperature for planets like Earth or Venus. These planets have an atmosphere or ocean that transport heat effectively, especially Venus with virtually no day-to-night or pole-to-equator temperature gradient. The atmosphere and/or ocean help smooth the diurnal temperature difference very well. Therefore, when coming up with a temperature estimate, it is a great first approximation. If you want the local equilibrium temperature for an airless body like Mercury or the Moon (that does not transport heat), then you want to use the no-redistribution or hemisphere only solar factor. This is well-known (see e.g., Selsis et al 2007). On Mercury, there is no heat distribution and very little thermal inertia; before the sunrise the temperature on the surface is somewhere near 100 K (-173 °C) and by noon the temperature on the surface of Mercury rises to about 700 K (427 °C). This may also be relevant for tide-locked planets (very slow rotation since one side is always facing the host star, the other in perpetual darkness). Earth does not experience any such changes of the sort. On Venus, the variability is even less and most of the planet is at around 735 K.
Summary
To summarize Part 1, Joseph E. Postma did not like a simple model of Earth’s radiative balance where we approximate the Earth as a sphere with uniform solar absorption. Of course, this is never done in climate modeling or in more detailed analyses appropriate for scholarly literature, so it is more an exercise in complaining about undergraduate education than an attempt to correct what he calls a “paradigm” in climatology. Nonetheless, the 0-D energy balance model is a useful approximation on Earth when coming up with an average emission temperature (~255 K), since air circulations and oceans tend to even out the diurnal temperature gradient on Earth, in addition to the thermal inertia provided by the system.
In Part 2, I will examine several of the other claims in the paper.
These posts comprise the Advanced rebuttal to Postma disproved the greenhouse effect































 Arguments
Arguments






























The problem is that much of what is believed about the greenhouse effect is a matter of interpretation. The intent is to interpret the data in the terms of making back-radiation/trapping responsible for the temperature found at the surface. However, these interpretations can be rejected out of hand immmediately, since there are other non-interpretatable factors which already lend to a higher surface temperature. Such as latent heat, which factually keeps the surface warmer than otherwise; and the natural lapse rate gradient, which as a matter of mathemtical fact will make the bottom of the atmosphere warmer than its average and more so than its top; and the actual real-time solar input, which is factually quite hot indeed, as the referenced hot-box experiments have demonstrated above, can directly induce temperatures well above 100C (of course which fills of the latent heat energy storage banks in liquid and vaporous water).
Thus, there are indeed material and factual objections which clearly relegate the back-radiation/trapping hypothesis as defunct, as there are actual factors which already lend to a higher bottom-of-atmosphere temperature.
"sufficient to emit greater energy at source than is recieve by incident radiation on the device"
That violates the first law of thermodynamics, and though I've refrained from engaging in the type of ad-hominem attack continually thrown my way, a statement like this really does expose scientific incompetency and a clutching of straws. I do apologize for having to make that remark, but alas, it couldn't be passed over in kindness, this time. Those preeminent experimentalists did not indeed interpret that their apparatus was magically producing more energy than received, ostensibly finding an exception to the 1st Law of thermodynamics. They would have laughed at that. What they found is that they could get sunlight to induce its maximum temperature on a plane, a temperature which is well above +100C. It is not an unexpected result.
Here for example is data (from this paper) which shows a measured solar flux higher than 1000 W/m^2.
Due to orbital eccentricity, the solar constant can actually be as high as ~1410 W/m^2, or +124C. That paper went on to show that the temperature induced at the surface was not actually bumped up to a higher temperature from back-radiation/trapping, and it also showed that the surface cools faster overnight than expected from a calculation without any back-radiation/trapping.
In any case, with all of these substantial errors and claims for the effects of back-radiation/trapping, which other physical effects are already at least partly if not entirely responsible for, it is simply sensible for me as a scientist to rework the problem without the usual interpretation and pre-interpretations typically applied, which of course as has been discussed, can logically be discarded outright in any case. Thanks for the discussion.
JPostma @45.
I think your response demonstrates quite conclusively that my dreadful feeling was fully justified.
Now this strange interpretation of the laws of thermdynamics you present - let's try to get more of a grasp of what you propose.
You seem happy to accept that a body will not emit any photons that would have been destined to be absorbed by a body with a higher temperature. So if I have two bodies, say a cold lump of iron and a hot furnace, I pop the iron in the furnace and the hot furnace will radiate photons at the iron as per standard physics. I assume you are signed up to that. But in your version the iron will radiate nothing whatsoever at the furnace. Not a single photon.
Time passes and the iron is soaked in the furnace and the furnace is then switched off, now quickly becoming cooler than the hot iron. So my question - Is there an instantaneous point in time when the iron that has now become hotter than the cooling furnace starts radiating as per standard physics and the furnace now cooler stops radiating? Or are you proposing a more gradual transformation from emitting to absorbing? (I assume here, for simplicity's sake, the surfaces of both bodies have each a uniform temperature as they warm/cool - so no warm spots/cool spots on the iron or on the walls of the furnace.)
The lump of iron inside the furnace will not make the furnace hotter, indeed as the lump of iron radiates what is required of it.
The argument is of course a strawman, assuming certain illogicities in what is desired to interpret.
This is all very simple but for the expedient of the apparent requirement to discuss it - The lump of iron and the funace can come to equilibrium, the temerature of the iron being determined by the heating source, the furnace. If the furnace provides an internal isotropic temperature, then the iron will come to that temperature in due time. When the furnace is shut off, then the furnace will cool, and so will the iron.
JPostma I strongly recommend that you give a direct answer to MA Roger's thought experiment. Such thought experiments are an excellent way of demonstrating who is right, but I have noticed in discussions of climate change as distinct unwillingness to properly engage with clear thought experiments and they are all too often met with evasion and Gish gallops.
I somehow doubt you will be able to give an answer to this question; one of the real advantages of the modern statistical interpretation of thermodynamics is that no mechansim is needed to avoid photons being emitted from a cooler object in the direction of a warmer one. Ockams' razor suggests that if two theories explain the observations equally well, choose the simpler of the two. Statistical thermodynamics is clearly simpler than one in which any tansfer of energy from a cooler object to a warmer one is prohibited, and explains all observations equally well.
It seems that JPostma replied to MA ROgers thought experiment while I was writing my post at 54; however I note that he did not actually answer the question posed, which was stated perfectly clearly:
I also have a thought experiment that I would like to discuss with JPostma, but to avoid "dogpiling", I will wait until the discussion of MA Rogers' thought experiment is concluded.
Actually I pointed out that the thought experiment illogically assumed a certain condition, such as to set up a straw man. Thus the question can have no direct answer.
Subsequently however, I described the process of heating the iron inside the furnace, which I will repeat:
The lump of iron and the funace can come to equilibrium, the temerature of the iron being determined by the heating source, the furnace. If the furnace provides an internal isotropic temperature, then the iron will come to that temperature in due time. When the furnace is shut off, then the furnace will cool, and so will the iron.
Joe, does the iron lose any energy while the furnace is on? If so, how?
The iron and the furnace can come to equilibrium, at which point the iron has attained the temperature supplied by the furnace...assuming an idealized system of unit absorption and emission, etc. etc.
While at equilbirium, the iron is not losing energy - its energy is constant, or else it would be changing temperature. There is an equal exchange between emission and absorption at equilibrium.
JPostma, the fact that you think that the thought experiment is a straw man may be because you do not understand the point of it. That doesn't mean the point doesn't exist, and a good scientist would just answer the question as posed, and not worry about giving a hostage to fortune (because if they were wrong, they would actively want to be corrected).
You are also wrong about the iron coming into equilibrium with the furnace, that is only true in the trivial case that they had both equilibriated to the ambient temperature, and is ignoring the fact there would be a thermal gradient until that had ocurred (neatly evading answering the question directly).
I'm sorry, I for one am not impressed by evasion. If you want to convince others, you will need to be willing to give direct, candid answers to direct questions.
Joe: "While at equilbirium, the iron is not losing energy - its energy is constant, or else it would be changing temperature. There is an equal exchange between emission and absorption at equilibrium."
An equal exchange: the iron is emitting and absorbing at equilibrium. It is losing energy and gaining energy at equilibrium. Do you also agree that there is an exchange of energy taking place during the heating process (but the net result is that the iron is a gainer)?
You had assumed certain statements about non-emission from the iron, thus setting up a strawman.
Indeed, the iron can do nothing but be compelled (due to energy absorption) to rise in temperature to that being supplied by the furnace, at which point the temperature of the iron will stop rising, and come to equilibrium with the source furnace. Indeed there is a thermal gradient between the iron and the furnace and this is why the iron is induced, by heat flow, to rise in temperature until it equilibrates with the furnace. These are direct answers and I am sorry if you found them to be evasive.
JPostma the question as posed was
Note the question is about when the iron starts radiating. You still have not answered that question, as you have not said when the iron starts to radiate.
DSL, such things are abvious, and indeed stated if not implied in my explanation of why the iron gets heated by the furnace.
However, to be sure, the "exchange of energy" during the heating process is, to state precisely, an exchange of heat, which is into the iron only. The cold iron does not heat the furnace. We have iron-ore smelters, and those workers do not shovel iron ore into the smelting furnace in order to make the furnace hotter - they shovel in coke, coal, or whatever, etc.
"you have not said when the iron starts to radiate"
I have stated in my first response, to quote: "the lump of iron radiates what is required of it". This is of course how it can come to equilibrium, how the state of zero heat flow can be established.
If the furnace somehow cooled down faster than the iron, then the iron would suppy some heat to the furace and slow down the furnace's cooling from inside, but it would not raise the furnace's temperature, of course, and soon, both items would cool to ambient.
JPostma wrote "I have stated in my first response, to quote: "the lump of iron radiates what is required of it"."
This is very obviously an evasive non-answer as it avoids specifically stating when the iron starts radiating (which was the question) and instead leaves the answer at best only implied. This sort of thing is not acceptable behaviour in a scientific discussion (as the point of the thought experiment was presumably to explicitly discuss the implications of your position).
"If the furnace somehow cooled down faster than the iron,"
you say "somehow" as if to suggest this is an unlikely scenario. It clearly isn't. The iron in the furnace (as we are discussing radiative transfer) is likely to be insulated (if only by a vacuum) from the body of the furnace, so if the body of the furnace is cooled after the iron has equilibriated with it, then of course the iron will cool more slowly.
You still have not unambiguously answered MA Roger's question (at what point would the iron start radiating, hint you need to specify a point in time). The fact that it is taking so long to answer such a simple question should be a cause of some concern to you.
"However, to be sure, the "exchange of energy" during the heating process is, to state precisely, an exchange of heat, which is into the iron only."
It seems that you do not understand the meaning of the word "exchange". There is no such thing as a unidirectional exchange. An exchange necessarily implies a transfer in both directions, although there may be a restriction on the net transfer.
Appologies to MA Roger for intruding into the discussion of his thought experiment; I shall drop out now.
Joe: "The cold iron does not heat the furnace."
The cold iron does not radiate? If it radiates, then the net exchange is simply altered. The exchange never stops. Does the temperature of the iron in the initial state matter for the temperature of the furnace? Yes, Joe. Yes it does. A frozen turkey in my oven will be more of a net gainer of energy than a thawed turkey. I'll end up using more energy to bring the frozen turkey to 60C than I will with a thawed turkey.
In other words, it's all relative. The thawed turkey has heated the oven relative to a condition with a frozen turkey.
As words seem to creating only more confusion, perhaps the maths of heat flow will help? Heat flow in an ideal radiative situation is Q = sigma*(Tf^4 - Ti^4) where Tf is the temperature of the furnace and Ti is the temperature of the iron. Equilibrium is acheived when Q = 0, i.e. when heat flow equals zero, when there is no more heat flow. This means that the iron is not gaining or losing any energy but has constant energy. The end point is that Ti = Tf, the iron has come to the temperature supplied by the furnace. Of course, heat flow is only from the furnace into the iron. If the furnace was shut off and cooled faster than the iron, then the iron could heat the furnace somewhat and slow what would have otherwise been its cooling rate.
The question as to "when" the iron starts to radiate was what was precisely set up as the strawman. If you truly require me to answer "when" the iron starts to radiate, it is troublesome. But the answer is obvious, it has been stated, and the answer is specified directly in the heat flow equation here, and also by the simple understanding of heat flow and the ability to attain equilibrium.
"I'll end up using more energy to bring the frozen turkey to 60C than I will with a thawed turkey."
The cold turkey, of course, does not heat the oven. A colder turkey takes more time to heat, indeed, but neither a cold turkey nor a colder turkey heats up the oven.
JPostma wrote "If you truly require me to answer "when" the iron starts to radiate, it is troublesome.",
Well yes, the fact that it is troublesome under your understanding of thermodynamics is exactly the point. It isn't at all troublesome for the modern statistical view of thermodynamics. The fact that you can't state the answer to such a straightforward question, and have to resort to such transparent equivocation, really says it all, and should give you pause for thought (to say the least).
Sorry again MA Roger, it was a very well thought out question. I suspect though that JPostma will be unable to see that the fact it is troublesome is a sign that his understanding of thermodynamics is decades out of date.
Here is the answer, in words: "The iron will radiate what is required of it". I am sorry, I thought you might appreciate the meaning of that, but I suppose I shouldn't do that.
So, if you look at the equation, Q = sigma*(Tf^4 - Ti^4), it is precisely clear when the iron radiates. It couldn't be any more clear. I am sorry if it is not clear to you. The answer is "as soon as the iron has a temperature". Thus, the iron can come to equilibrium with the source furnace, which is acheived when Q = 0, and of course, the heat flor equation shows that heat only flows from the furnace and into the iron while the iron is being heated; the iron will thus not heat the furnace, of course.
JPostma, repeating "The iron will radiate what is required of it" doesn't make it any less evasive.
Lets try an even more simple thought experiment. Consider a spherical blackbody object (A) at a temperature slightly above absolute zero, in a total vacuum with no other sources of radiation. Do you agree that the object will radiate photons in random directions according to the Stefan-Boltzmann law? A "yes" or "no" answer ought to be possible here, if "no" please state this explicitly and explain why.
I have just stated the answer, directly. I am sorry if you missed it again.
Please familiarize yourself with the heat flow equation Q = sigma*(Tf^4 - Ti^4). Is the answer still not clear? It also answers your latest question. Please let me know if you still require my help with this, and I will try to make it clear. An object radiates power P = A*sigma*T^4 where A is the surface area and T is the temperature. If you have two objects in a simplified geometry then you get the heat flow as the difference between their emissions, with heat flowing only from the warmer to the cooler. I hope this helps.
JPostma, I will not rise to your ineffectual attempts to be patronising ("Please let me know if you still require my help with this"), as I said the question is very easily answered under the modern statistical intepretation of thermodynamics, the fact that you can't (or won't) give a direct answer to MA Roger's question says it all.
Now, perhaps you would like to answer my question (it is the start of a thought experiment, but I thought it would be best to establish something we both ought to be able to agree on as a solid foundation)?
All of the questions have been answered. I am honestly trying to help you guys out, because it seems to be clear that a few basic thermodynamics and physics concepts might be unknown to you. I truly do not mean to sound patronizing or anything like that in making that statement, that is not my intention at all. So I do apologize for that. I have simply been assuming a certain level of ability to understand physics and thermodynamic concepts, such as the basic equation for heat flow under radiant emission, equilibrium conditions, etc.
The lump of iron is heated by the furnace. The iron does not heat the furnace. The heat flow equation shows exactly how and when the iron is radiating, as would the most basic conceptual understanding of the physics stated in words.
I hope this helped, and again, apologies for assuming certain things to be understood.
"All of the questions have been answered. "
no, you have not answered mine, posed at 71, here it is again for your convenience
This ought to be uncontentious, so you should just be able to reply "yes", in which case we can move on to the next step.
I did answer that precisely, in fact, to quote:
"An object radiates power P = A*sigma*T^4 where A is the surface area and T is the temperature. If you have two objects in a simplified geometry then you get the heat flow as the difference between their emissions, with heat flowing only from the warmer to the cooler."
Perhaps I am giving too much information, and it distracts you from being able to infer the answer. The answer, is of course, and I am sorry if you do not see the answer in the physics and in the math, that yes, and object radiates power as function of its surface temperature. I do hope this helps, and I hope that you can begin to recognize the greater answer in the math and physics.
I have used the Stefan-Botlzmann law to give you the answer, as you referenced it, so I am sorry if it was missed within the body of text.
JPostma, why not simply reply "yes", rather than equivocate? You are giving the impression of trying to avoid discussing the thought experiment by being unwilling to give simple direct answers to simple direct questions. There is no need for equations for such a simple question where "yes" fully answers the question.
So, is it a "yes" or a "no"?
Dikran, I am afraid that the equivocation is on your part, as you refuse to acknowledge the direct answer, and even the simple "yes" as you have requested it, which I have supplied to you. This seems to be some game you are playing now, and have been for some time in fact. I am sorry that I do not wish to play such a simple thing with you. I have in fact more fully answered the question than stating "yes", even though I have statedd "yes", by supplying the actual Stefan-Boltzman equation, which of course you had referenced initially. I am sorry if these satisfactions still do not fulfill you.
JPostma wrote "even though I have statedd "yes", by supplying the actual Stefan-Boltzman equation, which of course you had referenced initially"
supplying the Stefan Boltzmann equation does not equate to an unambiguous answer of "yes" as my question also involved the direction in which the photons were radiated, which is not covered by the Stefan-Botzmann law (only the power). So despite the amount of words that have been written, you still have not answered my question, which was:
Note I have highlighted the part of the question you have not yet addressed to make sure you don't miss it this time.
This is not a game (at least on my part). The reason for wanting a direct answer was to make sure there could be no misundertanding of your position. "yes" and "no" are utterly unequivocal, which is why I designed my question to be answerable with a simple "yes" or "no".
So please, for the record, is your answer to my question (including the bit about the direction in which photons are radiated) "yes" or "no"?
Of course, thermal radiant emission goes out in all directions not otherwise blocked to it. I am glad that we can state such basic physics concepts.
As the example you presented was of a sphere in space, then the radiant emission power is given by P = A*sigma*T^4, and this goes out into space in all directions, as a uniform wavefront basically.
JPostma, please give a simple "yes" or "no" answer to questions where this is possible, in order to avoid ambiguity. I will take that answer as a "yes".
Now consider a second black body object "B" which is introduced into the scenario. B is a plane of infinite extent and infinite conductivity (so it is the same temperature everywhere). B is maintained at a temperature fractionally higher than that of A. B is placed to the right of A, and in close proximity, but not actually touching, so the only form of heat transfer possible will be radiative.
Now, as B is warmer than A, is it your contention that A will not radiate any photons that will strike B (i.e. A will only emit photons to its left and none to its right)?
This is a question where a "yes" or "no" answer is possible. If "yes", then no further comment is necessary as we understand eachother; if "no", please clearly state that your answer is "no" and then provide an explanation.
We simply apply the heat flow equation using the Stefan-Boltzmann Law. The result is that heat flow is from hotter to cooler, of course. Thus, the cooler object heats up. The cooler one does not heat the warmer one, of course, and the equation for heat flow obviously does not imply this. Equilibrium is given when the respective emissions cancel eachother out, as in Q = sigma*(Tf^4 - Ti^4). Photons are of course emitted and the equation does not say that no photons are emitted from the cooler object, as you have attempted to conjecture. It is the balance of emission which determines equilibrium, not lack of emission.
Again I do apologize for answering with more information, but it is necessary to simply and kindly provide clarification of the physics, such that some erroneous interpretation that a cold source can heat a warm source is prevented from being assumed or inferred, which would of course be ridiculous in any case.
JPostma, yet again you avoid giving a straightforward "yes" or "no" answer, so I shall rephrase it.
Does A emit any photons that strike B, "yes" or "no"?
There can only be two answers to this question, either it does or it doesn't, so any unwillingness to give a straight "yes" or "no" will be an indication of deliberate obstruction on your part, as far as I am concerned.
The link to J.Postma's paper in the OP appears broken. Considering how confident Mr Postma is of the need for everyone here to receive his teaching on Thermodynamics, I was curious to take a look at it and check the science publication where it appeared.
I am very surprised by this statement: " the expected results of the artificial radiative greenhouse effect, using any argument or model for it, do not manifest, thus confirming their artificiality."
The radiative transfer models do predict with remarkable accuracy not only the downwelling IR at the surface (both quantitatively and in wave length distribution) of which real time measurements are easily accesible for some locations, but at various other altitudes too. What data is there to support the statement that the expected results do not manifest?
I am offering you as much information as I possibly can. The answer is stated precisely by the heat flow equation which I have given you.
Let me repeat:
"As the example you presented was of a sphere in space, then the radiant emission power is given by P = A*sigma*T^4, and this goes out into space in all directions, as a uniform wavefront basically."
and
"We simply apply the heat flow equation using the Stefan-Boltzmann Law. The result is that heat flow is from hotter to cooler, of course. Thus, the cooler object heats up. The cooler one does not heat the warmer one, of course, and the equation for heat flow obviously does not imply this. Equilibrium is given when the respective emissions cancel eachother out, as in Q = sigma*(Tf^4 - Ti^4). Photons are of course emitted and the equation does not say that no photons are emitted from the cooler object, as you have attempted to conjecture. It is the balance of emission which determines equilibrium, not lack of emission."
'A' emits photons in all directions. However, emission from 'A' does not heat a warmer object, as the heat flow equation shows that heating only occurs from hot to cold.
I do sincerely hope this is clear for you.
See the later paper here @PC. A higher temperature than the maximum solar insolation temperature is not observed...which means that back-radiation/trapping does not contribute to surface heating.
JPostma, I'm sorry, you are being deliberately obstructive. I could not have taken more effort in posing the question for you in a way that made a simple, direct, unequivocal, unambiguous answer possible, but yet again you refuse to do so.
Here is your last chance to show that you are genuinely engaged in this discussion and are not just obfuscating:
Does A emit any photons that strike B, "yes" or "no"?
If your answer is anything other than "yes" or "no", it will be a tacit admission on your part that you are being deliberately obstructive.
I have precisely stated the answer for you Dikran. This is a silly game you play...as if you wish to move my mouth for me, or some dominance thing. It is silly and not necessary.
'A' emits photons in all directions. However, emission from 'A' does not heat a warmer object, as the heat flow equation shows that heating only occurs from hot to cold.
I am sorry if you wish to attempt to find a different answer than this, but this is the answer. You can not deny the Stefan-Boltzmann Law heat equation. Are things clear for you yet, on the physics? Do you wish to rather simply state your desired end-point, rather than directing me how to move my mouth for you?
[TD] JPostma, you are refusing to answer simple questions with simple answers. You are merely repeating your statements regardless of the question, which constitutes sloganeering, which violates the rules of engagement for this site.
JPostma, it is a shame you take that attitude. Had you just replied "yes", you would have doen yourself a big favour as it would have revealed where an apparent misunderstanding about your position has arisen. Your loss entirely.
"yes" is only three letters, you should ask yourself why you were unwilling to write them, rather than carry on with this obstructive behaviour.
Emission from 'A' in all directions equates precisely to 'yes' how is that not clear? But it is of course important to follow that up with some physics, such as the Stefan-Boltzmann Law and heat flow equation, which indicates that emission from the cold object will not warm the warmer object.
I find it surprising that there is an assumption that Dikran is the director of this conversation and that I must state answers only in the way he or she desires them - there is of course no point in conversation if my role here is only to say what Dikran requests of me.
I am dissapointed both at Dikran and the moderator for thus prejudicial behaviour. I had thought to be free to discuss physics.
This is clear indeed, and rather boring. This is just a repeat of G&T nonsense and playing on words to spread confusion. We've talked at length about this years ago. Net heat transfer is different from energy transfer. Energy, under the form of photons, can be transferred from any object to any other object, regardless of temperature, as long as it is above absolute zero. Net heat, which in Thermodynamics is usually referred to as just "heat" can only go from hot to cold.
Postma's condescending tone does not match his unability to answer the very simple question posed by Dikran. Thers is no need to give additional information or "drown the fish" in a sea of words. The thought experiment described by Dikran is simple. The question is does A emit photons that strike B, not does A heat B or does A emit photons. The answer from Postma should be yes or no.
So, Mr Postma, for the last time, in the thought experiment described above, does object A emit any photon that strikes object B? Anything else than yes or no will be considered obfuscation by me.
Q = sigma*(Th^4 - Tc^4) shows quite precisely that emission from a cold source does not heat up a warmer source. The emission from the cold source is what balances with the emission from the warm source in order to determine equilibrium - the emissions cancel out, leaving equilibrium, whether or not we wish to discuss whether the photons from the cold source "strike" the hot source...there is a greater physics occuring than that, as found in the heat equation, which shows how emission actually balances or cancels out at equilbrium.
JPostma, I had repeatedly asked you just to reply "yes" or "no" to avoid all ambiguity, you chose not to, even when doing so would be a tacit admission that you were being deliberately obstructive. I am always happy to discuss science, and be proven wrong if that is what I am, however I don't have endless enthusiasm for obfuscation, and I don't think it unreasonable for people to give a direct "yes" or "no" answer when explicitly asked for it.
You have missed your chance to clearly explain your position. My thought experiment was designed to very clearly distinguish between two possible explanations of your position, but you chose not to co-operate.
Being willing to give direct answers to direct questions should be taken for granted in a scientific discussion, where the aim is to determine the truth. The fact that you needed input from the moderator should tell you that your behaviour was unproductive. Note the moderator did not delete your post, just gave advice.
I see the answer cam while I was writing my post. That yes has numerous consequences, which essentially negate your argument. "Following it up with Physics" is a fancy name for obfuscation. The condescending tone is rather amusing. I'm done here, that yes was all I needed to measure the depth of Mr Postma's need to receive Physics education, not give it
"So, Mr Postma, for the last time, in the thought experiment described above, does object A emit any photon that strikes object B? Anything else than yes or no will be considered obfuscation by me."
The problem is that answering only with a "yes or no" leaves the door open to misterpretation and sophist obfuscation of the physics, because without the heat flow equation the meaning of the answer can be interpreted arbitrarily. Thus, I include the heat flow equation to help make it clear what the result of the physics is: heat not flowing from cold to hot, which means the warmer source does not increase in temperature.
JPostma writes "The problem is that answering only with a "yes or no" leaves the door open to misterpretation and sophist obfuscation of the physics"
Sorry, but that is hilarious. An answer of "yes" to the question "Does A emit any photons that strike B, "yes" or "no"?" leaves the door open to misunterpretation? Give me a break! Better still, give me an example of how it could be misinterpreted!
More than avoid ambiguity, which is what would occur with a simple "yes or no" answer, I have provided the heat flow equation and some discussion of it to help clarify the fact that opposing emission is what determines equilibrium, and that the warmer object will not be heated, i.e., raise in temperature, due to the cooler object. I am sorry that I can not provide ambiguous answers, and that my explanation of the answer seems like ambiguity to you. But it is simple: cold does not heat hot, opposing emission determines equilibrium, etc.