Stratospheric Cooling and Tropospheric Warming
Posted on 1 December 2010 by Bob Guercio
This post has been revised at Stratospheric Cooling and Tropospheric Warming - RevisedIncreased levels of carbon dioxide (CO2) in the atmosphere have resulted in the warming of the troposphere and cooling of the stratosphere. This paper will explain the mechanism involved by considering a model of a fictitious planet with an atmosphere consisting of carbon dioxide and an inert gas such as nitrogen at pressures equivalent to those on earth. This atmosphere will have a troposphere and a stratosphere with the tropopause at 10 km. The initial concentration of carbon dioxide will be 100 parts per million (ppm) and will be increased instantaneously to 1000 ppm and the solar insolation will be 385.906 watts/meter2. Figure 1 is the IR spectrum from a planet with no atmosphere and Figures 2 and 3 represent the same planet with levels of CO2 at 100 ppm and 1000 ppm respectively. These graphs were generated from a model simulator at the website of Dr. David Archer, a professor in the Department of the Geophysical Sciences at the University of Chicago and edited to contain only the curves of interest to this discussion. The parameters were chosen in order to generate diagrams that enable the reader to more easily understand the mechanism discussed herein.
Prior to discussing the fictitious model, consider a planet with no atmosphere. In this situation light from the sun that is absorbed by the surface is reemitted from the surface. Figure 1 is the IR spectrum of this radiation which is known as Blackbody radiation.
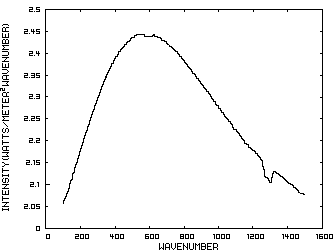
Figure 1. IR Spectrum - No Atmosphere
Consider now Figure 2 which shows the Infrared (IR) radiation spectrum looking down at the planet from an altitude of 10 km with a CO2 concentration of 100 ppm and Figure 3 which shows the IR spectrum with a CO2 concentration of 1000 ppm. Both figures represent the steady state and approximately follow the intensity curve for the blackbody of Figure 1 except for the missing band of energy centered at 667 cm-1. This band is called the absorption band and is so named because it represents the IR energy that is absorbed by CO2. IR radiation of all other wavenumbers do not react with CO2 and thus the IR intensity at these wavenumbers is the same as that of the ground. These wavenumbers represent the atmospheric window and is so named because the IR energy radiates through the atmosphere unaffected by the CO2. The absorption band and the atmospheric window is the key to stratospheric cooling.
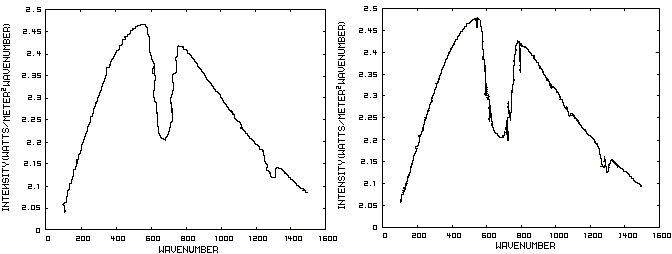
Figure 2. CO2 IR Spectrum - 100ppm Figure 3. CO2 IR Spectrum - 1000 ppm
The absorption band in Figure 3 is wider than that of Figure 2 because more energy has been absorbed from the IR radiation by the troposphere at a CO2 concentration of 1000 ppm than at a concentration of 100 ppm. The energy that remains in the absorption band after the IR radiation has traveled through the troposphere is the only energy that is available to interact with the CO2 of the stratosphere. At a CO2 level of 100 ppm there is more energy available for this purpose than at a level of 1000 ppm, thus the stratosphere is cooler for the higher level of CO2 in the troposphere. Additionally, the troposphere has warmed because it has absorbed the energy that is no longer available to the stratosphere.
One additional point should be noted. Notice that the IR radiation in the atmospheric window is slightly higher in Figure 3 than Figure 2. This is because the temperature of the troposphere has increased and in the steady state condition, the total amount of IR entering the stratosphere in both cases must be the same. That total amount of energy is the area under both of these curves. Thus, in Figure 2, there is more energy in the absorption band and less in the atmospheric window while in Figure 3, there is less energy in the absorption band and more in the atmospheric window.































 Arguments
Arguments























 0
0  0
0 Figure 1. IR Spectrum - No Atmosphere
The curves of Figures 2 approximately follow the intensity curve of Figure 1 except for the missing band of energy centered at 667 cm-1. This band is called the absorption band and is so named because it represents the IR energy that is absorbed by CO2. IR radiation of all other wavenumbers do not react with CO2 and thus the IR intensity at these wavenumbers is the same as that of Figure 1. These wavenumbers represent the atmospheric window which is so named because the IR energy radiates through the atmosphere unaffected by the CO2.
Figure 1. IR Spectrum - No Atmosphere
The curves of Figures 2 approximately follow the intensity curve of Figure 1 except for the missing band of energy centered at 667 cm-1. This band is called the absorption band and is so named because it represents the IR energy that is absorbed by CO2. IR radiation of all other wavenumbers do not react with CO2 and thus the IR intensity at these wavenumbers is the same as that of Figure 1. These wavenumbers represent the atmospheric window which is so named because the IR energy radiates through the atmosphere unaffected by the CO2.
 Figure 2. CO2 IR Spectrum - 100/1000 ppm
A comparison of the curves in Figure 2 shows that the absorption band at 1000 ppm is wider than that at 100 ppm because more energy has been absorbed from the IR radiation by the troposphere at a CO2 concentration of 1000 ppm than at a concentration of 100 ppm. The energy that remains in the absorption band after the IR radiation has traveled through the troposphere is the only energy that is available to interact with the CO2 of the stratosphere. At a CO2 level of 100 ppm there is more energy available for this than at a level of 1000 ppm. Therefore, the stratosphere is cooler because of the higher level of CO2 in the troposphere. Additionally, the troposphere has warmed because it has absorbed the energy that is no longer available to the stratosphere.
In concluding, this paper has explained the mechanisms which cause the troposphere to warm and the stratosphere to cool when the atmospheric levels of CO2 increase. The dominant mechanism involves the conversion of the energy of motion of the particles in the atmosphere to IR radiation which escapes to space and the second method involves the absorption of IR energy by CO2 in the troposphere such that it is no longer available to the stratosphere. Both methods act to reduce the temperature of the stratosphere.
*It is recognized that a fictitious planet as described herein is a physical impossiblity. The simplicity of this model serves to explain a concept that would otherwise be more difficult using a more complex and realistic model.
Copyright 2010 - Robert J. Guercio
Figure 2. CO2 IR Spectrum - 100/1000 ppm
A comparison of the curves in Figure 2 shows that the absorption band at 1000 ppm is wider than that at 100 ppm because more energy has been absorbed from the IR radiation by the troposphere at a CO2 concentration of 1000 ppm than at a concentration of 100 ppm. The energy that remains in the absorption band after the IR radiation has traveled through the troposphere is the only energy that is available to interact with the CO2 of the stratosphere. At a CO2 level of 100 ppm there is more energy available for this than at a level of 1000 ppm. Therefore, the stratosphere is cooler because of the higher level of CO2 in the troposphere. Additionally, the troposphere has warmed because it has absorbed the energy that is no longer available to the stratosphere.
In concluding, this paper has explained the mechanisms which cause the troposphere to warm and the stratosphere to cool when the atmospheric levels of CO2 increase. The dominant mechanism involves the conversion of the energy of motion of the particles in the atmosphere to IR radiation which escapes to space and the second method involves the absorption of IR energy by CO2 in the troposphere such that it is no longer available to the stratosphere. Both methods act to reduce the temperature of the stratosphere.
*It is recognized that a fictitious planet as described herein is a physical impossiblity. The simplicity of this model serves to explain a concept that would otherwise be more difficult using a more complex and realistic model.
Copyright 2010 - Robert J. Guercio
 You will notice the small spike at the center of the CO2 absorption/emmision pattern in each case. That spike represents emissions by stratospheric CO2, which being warmer than stropospheric CO2, has a higher brightness temperature. The important point to notice is that the spike is confined to the center of the CO2 absorption/emmision band.
Looking at your figure 2 brought my attention to the fact that the majority of excess absorption in the troposphere with increased CO2 takes place on the wings of the band, not at the center. It appears from your figure 2 that there is no reduction in CO2 absorption at the center of the band. But because it is at the center of the band where stratospheric CO2 emits and absorbs, it follows that the reduction in IR on the wings of the band will have no tendency to cool the stratosphere.
Clearly, in the non-equilibrium state, adding extra CO2 will reduce the brightness temperature at the center of the CO2 band as well as at the wings, and will consequently reduce stratospheric temperatures. But as the troposphere achieves radiative equilibrium, it may be that the loss of IR radiation on the wings of the band undercompensates for, exactly compensates for, or over compensates for the increased emmisions outside that band due to increased surface temperature. In the first case, the equilibrium brightness temperature at the center of the CO2 band will be less than it was before introducing more CO2, thus cooling the stratosphere. In the second, it will have no effect; and in the third it will slightly warm the stratosphere. As to which case will actually apply, you will have to ask an expert; and it may be the models insufficiently clarify the situation. For practical purposes though, it appears that the cooling of the stratosphere due to the second method is a temporary effect, which declines to close to zero as the atmosphere achieves radiative equilibrium.
(As an aside,the emission spectrum for Antarctica is especially interesting; showing, as it does, that the tropospheric CO2 was warmer than the surface. In the situation at the time of this observation, the effect of CO2 in the atmosphere would have been to cool the surface of Antarctica, rather than to warm it. ;) )
On to the first method:
Where you say, "... this vibration is related to the energy content of CO2, it is not related to the temperature of the gaseous mixture", this is not strictly correct. The energy stored as vibration is not measured by the temperature, but there is an equilibrium relationship between the heat stored as molecular vibrations and the temperature of the gass. The actual relationship varies from gas to gas, and depends of the degrees of freedom of their vibrational modes.
Because the relationship between heat stored as vibration, and heat stored as translation energy, adding more CO2 at the same temperature will not cool a gass (ignoring considerations of pressure and volume), for the added CO2 will have the same proportion of energy stored as internal vibrations. Adding a cooler or warmer amount of CO2 will, of course, temporarilly cool or warm the stratosphere, but the stratosphere will quickly return to equilibrium.
What is happening in any gass is that the energy stored as vibration interacts with, and seeks to achieve equilibrium with two sources of energy. The first is the energy from collisions within the gass, which is a function of temperature. The second is the radiant energy it emits (which is a function of its temperature) and recieves (which is a function of the temperature of the source of the radiation it captures). If the temperature of the gas is less than the temperature of source of its radiant energy, its the energy it radiates will be less than that which it recieves, increasing its vibrational energy. This excess will then be passed onto the ambient gass, increasing its temperature. If the radiant energy it recieves has a lower "temperature" than the ambient gass, its will emit more energy than it recieves, draining its pool of vibrational energy. This shortfall will then be made up by collisions with other gass molecules, cooling the ambient gass.
Applying this to your model, and assuming all energy transfers are radiant, the effect is that the stratospheric gass will reach an equilibrium temperature equal to the brigtness temperature of the tropospheric CO2. If the stratosphere were cooler than that, than the stratospheric CO2 would be a net absorber of radiant energy, thus warming the stratosphere. If it were warmer, the CO2 would be a net emitter, thus cooling the stratosphere. (In reality, the temperature would be determined by convection and the adiabatic lapse rate, which would dominate at stratospheric altitudes were it not for a major source of radiant energy to those levels.)
So, once again, I come back to Ozone. where it not for Ozone being a net absorber of energy in the stratosphere, CO2 would not be a net emitter of energy in the stratosphere. And it is only by being a net emitter that CO2 can cool.
You will notice the small spike at the center of the CO2 absorption/emmision pattern in each case. That spike represents emissions by stratospheric CO2, which being warmer than stropospheric CO2, has a higher brightness temperature. The important point to notice is that the spike is confined to the center of the CO2 absorption/emmision band.
Looking at your figure 2 brought my attention to the fact that the majority of excess absorption in the troposphere with increased CO2 takes place on the wings of the band, not at the center. It appears from your figure 2 that there is no reduction in CO2 absorption at the center of the band. But because it is at the center of the band where stratospheric CO2 emits and absorbs, it follows that the reduction in IR on the wings of the band will have no tendency to cool the stratosphere.
Clearly, in the non-equilibrium state, adding extra CO2 will reduce the brightness temperature at the center of the CO2 band as well as at the wings, and will consequently reduce stratospheric temperatures. But as the troposphere achieves radiative equilibrium, it may be that the loss of IR radiation on the wings of the band undercompensates for, exactly compensates for, or over compensates for the increased emmisions outside that band due to increased surface temperature. In the first case, the equilibrium brightness temperature at the center of the CO2 band will be less than it was before introducing more CO2, thus cooling the stratosphere. In the second, it will have no effect; and in the third it will slightly warm the stratosphere. As to which case will actually apply, you will have to ask an expert; and it may be the models insufficiently clarify the situation. For practical purposes though, it appears that the cooling of the stratosphere due to the second method is a temporary effect, which declines to close to zero as the atmosphere achieves radiative equilibrium.
(As an aside,the emission spectrum for Antarctica is especially interesting; showing, as it does, that the tropospheric CO2 was warmer than the surface. In the situation at the time of this observation, the effect of CO2 in the atmosphere would have been to cool the surface of Antarctica, rather than to warm it. ;) )
On to the first method:
Where you say, "... this vibration is related to the energy content of CO2, it is not related to the temperature of the gaseous mixture", this is not strictly correct. The energy stored as vibration is not measured by the temperature, but there is an equilibrium relationship between the heat stored as molecular vibrations and the temperature of the gass. The actual relationship varies from gas to gas, and depends of the degrees of freedom of their vibrational modes.
Because the relationship between heat stored as vibration, and heat stored as translation energy, adding more CO2 at the same temperature will not cool a gass (ignoring considerations of pressure and volume), for the added CO2 will have the same proportion of energy stored as internal vibrations. Adding a cooler or warmer amount of CO2 will, of course, temporarilly cool or warm the stratosphere, but the stratosphere will quickly return to equilibrium.
What is happening in any gass is that the energy stored as vibration interacts with, and seeks to achieve equilibrium with two sources of energy. The first is the energy from collisions within the gass, which is a function of temperature. The second is the radiant energy it emits (which is a function of its temperature) and recieves (which is a function of the temperature of the source of the radiation it captures). If the temperature of the gas is less than the temperature of source of its radiant energy, its the energy it radiates will be less than that which it recieves, increasing its vibrational energy. This excess will then be passed onto the ambient gass, increasing its temperature. If the radiant energy it recieves has a lower "temperature" than the ambient gass, its will emit more energy than it recieves, draining its pool of vibrational energy. This shortfall will then be made up by collisions with other gass molecules, cooling the ambient gass.
Applying this to your model, and assuming all energy transfers are radiant, the effect is that the stratospheric gass will reach an equilibrium temperature equal to the brigtness temperature of the tropospheric CO2. If the stratosphere were cooler than that, than the stratospheric CO2 would be a net absorber of radiant energy, thus warming the stratosphere. If it were warmer, the CO2 would be a net emitter, thus cooling the stratosphere. (In reality, the temperature would be determined by convection and the adiabatic lapse rate, which would dominate at stratospheric altitudes were it not for a major source of radiant energy to those levels.)
So, once again, I come back to Ozone. where it not for Ozone being a net absorber of energy in the stratosphere, CO2 would not be a net emitter of energy in the stratosphere. And it is only by being a net emitter that CO2 can cool.
 And again with 1000ppm, total emission 11.0 W/m2
And again with 1000ppm, total emission 11.0 W/m2
 You can clearly see the increase in stratospheric emissions as CO2 rises. Ergo in equilibrium you'd expect T to decrease.
You can clearly see the increase in stratospheric emissions as CO2 rises. Ergo in equilibrium you'd expect T to decrease.







Comments