Fossil fuel-burning is acidifying the oceans and, up until recently, it has generally been thought that the greatest risk posed by ocean acidification was the change to seawater carbon chemistry. This is because rising levels of atmospheric carbon dioxide reduce the concentration of seawater carbonate ions, a vital building-block in the shells and skeletons of many marine life. Fish were not thought to be at direct risk from acidification, because they clearly don't build shells, and were considered to have well-developed physical mechanisms to tolerate falling pH (acidification).
Several studies published in the journal Nature Climate Change, Baumann (2011) and Frommel (2011), indicate that this might not be the case. Fish may, in fact, be seriously threatened by ocean acidification. Although adult fish seem well-equipped to deal with low pH waters, or higher levels of CO2 in seawater, their egg and larval life stages, a typically vulnerable time for all marine life, may not be so fortunate.
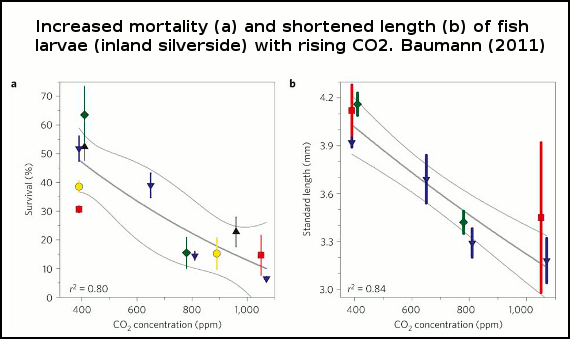
Baumann (2011) show that larvae survival in one fish species drops with increased levels of CO2 (figure 1). Survival rates plummeting some 75% under a scenario with 1000ppm (parts per million) of atmospheric CO2. And Frommel (2011) discovered considerable tissue damage and necrosis (dead tissue) in fish larvae of another species exposed to higher levels of CO2 than the present day. In the high CO2 experiments, this damage to internal organs was so extensive it lead to the death of afflicted larvae. Each of these studies are discussed in detail below.

Figure 1 -Survival rates of larvae with increasing CO2. Thin grey lines=error bars (95% confidence intervals). Experiment 1, red squares; experiment 2, blue down triangles; experiment 3, green diamonds; experiment 4, yellow circles; experiment 5, black up triangles. Points represent means±1 standard deviation (a spread of measurements deviating from the average).
It's important to point out that some regions of the oceans, especially coastal waters, can seasonally experience extremely acidified waters, with much lower pH than the global average. For example, due to upwelling over the warmer months, Kiel Fjord in the Western Baltic Sea (Germany) can see prolonged periods of acidification so low that it is equivalent to atmospheric CO2 levels of 2300 ppm. So this needs to be kept in mind when the reading the levels of atmospheric CO2 discussed in the following studies. High levels of ocean acidification are already occurring in some areas of the ocean where fish spawning fish takes place.
The inland silverside is an ecologically important small fish native to the Atlantic coast of North America, and is found in estuaries up-and-down the coast. It can also be found in acidified freshwater environments, and humans have even successfully transplanted them into land-locked freshwater systems and lakes, so they clearly can adapt to low pH (acidified) water.
Baumann (2011) conducted a series of experiments where CO2 was bubbled into seawater containing the newly-fertilized eggs of the inland silverside. Larvae were exposed to levels of acidification representative of modern-day (390-400ppm,) right up to projected late 21st century (900-1100ppm) atmospheric CO2 levels. After a week the surviving larvae were counted and measured.
In each of the 5 experiments both the larvae body length and survival rates declined as seawater became more acidified (figure 1), and there were also more deformed larvae (figure 2).
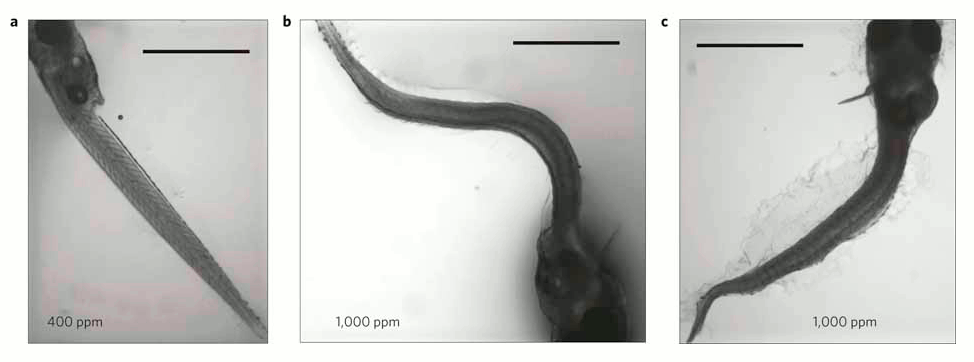
Figure 2- Larvae with curved or curled bodies were significantly more common at increased (b,c) when compared with control (a) CO2 levels. Scale bar=1 mm.
At 1000 ppm, survival plunged some 75% and larval body length 18%. The shorter length indicates a slower rate of growth, a problem for larvae because the longer the time they spend growing in their larval stage, the higher the rate of predation by plankton-eating fish and other critters. In other words, slower growth and larval deformities will likely translate into even lower survival rates in the wild, compounding the death rate of these larvae as ocean acidification increases.
The cause of this slow growth, deformation and decline in survival rates in the experiments is not known, but given that inland silverside can grow and thrive in more acidified freshwater, this suggests something other than pH. Baumann (2011) suggest this may be related to high CO2 levels, or even the carbonate chemistry of the water.
The authors speculated that the negative response may have been related to the susceptibility of very early larval development, the egg embryo, so carried out another experiment, After growing in seawater equivalent to 410 ppm for 5 days, fertilized eggs were exposed to 780ppm once they hatched. Survival rates only slightly dropped in this treatment versus the control (at constant 410 ppm), and survival was much higher than larvae exposed to 780 ppm the entire time, suggesting that it's the eggs themselves that may be vulnerable to ocean acidification.
Frommel (2011) was another experiment carried out on fish larvae, this time to focus was on Atlantic cod, a fish widely distributed throughout the North Atlantic Ocean, and one listed as vulnerable on the IUCN Red List of threatened species. This is the same species that was decimated by overfishing in the late 20th century, an activity which lead to the collapse of 6 populations off the Canadian coastline.
The cod larvae for this experiment were taken from a population off Norway, and were exposed to 3 levels of seawater acidity equivalent to 380 ppm (the control), 1800 ppm (medium) and 4200 ppm (high). Although these last two scenarios may seem very high, Kiel Fjord (noted earlier) is very near to Baltic cod spawning grounds and already sees levels above the equivalent of 1800ppm, and is likely to endure acidification comparable to around 4200 ppm with a doubling of atmsopheric CO2 (560 ppm). So the experiments are directly relevant to current and near-future conditions in which Atlantic cod spawn.
Cod larvae were reared for 7 weeks in large outdoor cylinders called mesocosms. The authors did not directly test for mortality (death rate), but instead looked for changes in tissue health. They discovered that in the early stages of development, the first 25 days after hatching was a critical time. Newly-hatched larvae lack fully-functioning gills, which is the primary organ for helping regulate internal pH balance and CO2 build up in tissue. In this early development stage the larvae are essentially at the mercy of the surrounding seawater pH, and are therefore very vulnerable. In the experiment, larvae suffered extensive damage to vital organs with rising acidity, and those larvae with extreme malformations to internal organs and widespead dead tissue, obviously died from these deformities. See figure 3.
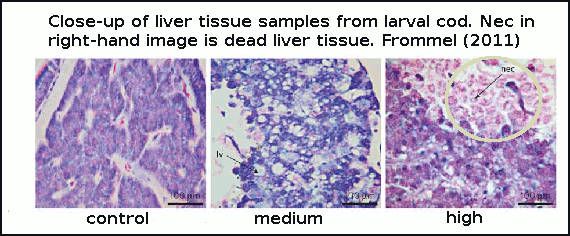
Figure 3 - histological sections (tissue samples under microscope magnification) of cod larvae liver at increasing CO2. Control=380ppm, medium=1800ppm and high=4200ppm.
The effect of the three acidity scenarios on larval tissue are is shown in figure 4. As acidity increases, so too does the amount of tissue damage.
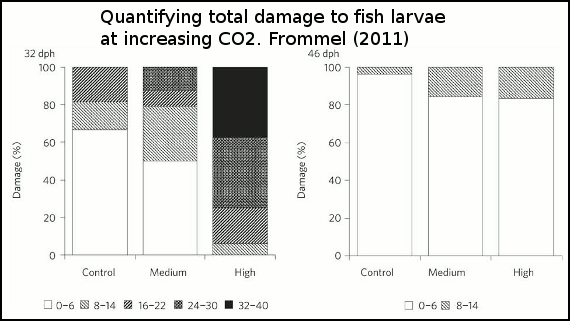
Figure 4 - Percentage of larvae exhibiting different degrees of total damage at 32 and 46 days after hatching and three different treatment levels (control, medium and high). The damage is shown as five levels with normal as white bars, and shading increasing with increasing severity of damage. Control=380ppm, medium=1800ppm and high=4200ppm.
In the summary of their paper the authors conclude:
"Although we did not directly test for mortality rates, our data on severe tissue damage suggest that ocean acidification will negatively impact the recruitment of mass-spawning fishes because of enhanced mortality rates."
To sum up:
Related SkS posts: OA not OK series, Ocean Acidification: Corrosive waters arrive in the Bering Sea, Acidification:Oceans past, present & yet to come, Ocean acidification: Some Winners, Many Losers, Ocean acidification: global warming's evil twin and Great Barrier Reef Part 3: Acidification, Warming, and Past Coral Survival.
Posted by Rob Painting on Thursday, 22 December, 2011
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |