Four of the “big five” mass extinctions, and several more minor environmental crises in Earth’s past, were associated with abrupt global warming and ocean acidification triggered by Large Igneous Province (LIP) eruptions. The most recent of the “big five” - the end-Cretaceous - was complicated by a large asteroid impact, but at least the marine extinction was triggered by the Deccan Traps LIP, as explained in this post. Yet not all LIPs were associated with environmental catastrophes. Some, like the early Cretaceous Paraná-Etendeka LIP, seem to have had very little effect on global climate.
So what made some LIPs destructive and others not? And, you may be asking, what possible relevance do these ancient events have for us today?
The answer is that they confirm what scientists have modeled from ocean chemistry: CO2 emission rates mattered back then just as they do now. In fact they are key.
I am focusing on the oceans because the oceans are a far, far larger reservoir of carbon than either the terrestrial biosphere or the atmosphere. The atmosphere today contains about 830 billion tonnes of carbon (GtC) (up from about 600 GtC before the industrial revolution), the terrestrial biosphere contains about 560 Gt, the surface layer of the ocean about 900 Gt and the deep ocean about 37,100 Gt. Humans have emitted about 600 Gt since the industrial revolution, and if we continue business-as-usual we might end up emitting as much as 1700 Gt by the end of the century, more than the terrestrial biosphere and surface ocean inventories combined. The oceans currently absorb about 30% of human CO2 emissions and 90% of the excess heat generated by modern climate change, but as terrestrial “sinks” for our CO2 emissions dwindle and even switch to becoming sources of CO2 this century (see this post), the oceans are fast becoming the main sink for absorbing our CO2 emissions.
Oceans are dynamic, allowing them to change in response to alterations in climate, but there are limits to that dynamism.
To understand why CO2 emission rates matter, you have to wet your feet in some basic ocean chemistry.

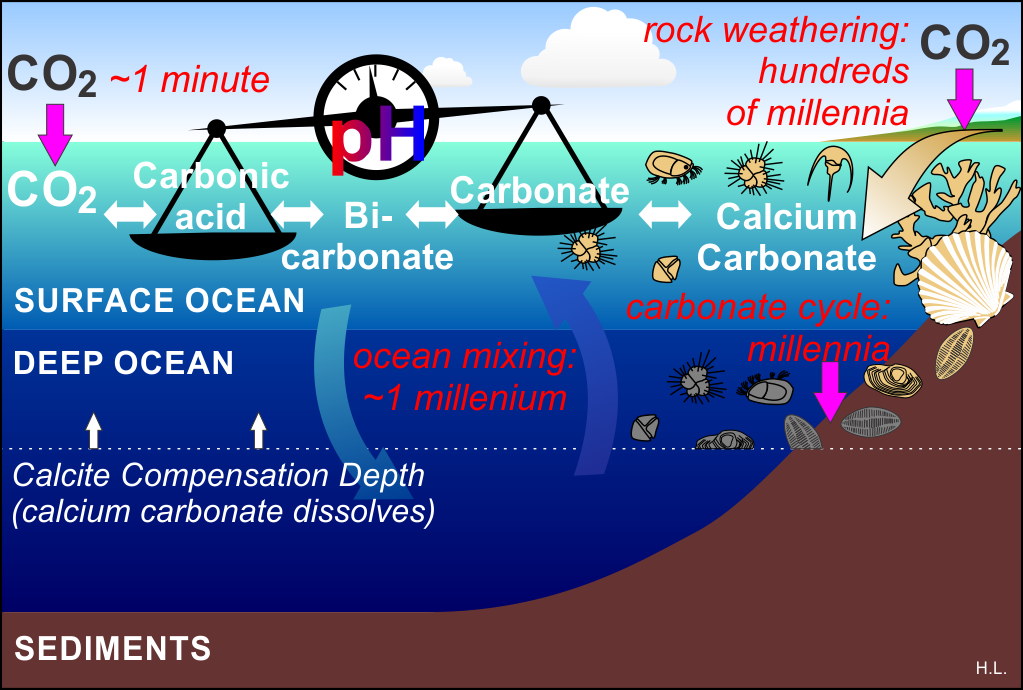
Figure 1: Rates of key aspects of ocean carbon chemistry in a time of rising atmospheric CO2 levels.
In a time of rising CO2 levels in the atmosphere, the surface layer of the ocean absorbs most (but not all) of that excess CO2 to regain balance with the atmospheric levels (Henry’s Law). Once dissolved in seawater, that CO2 reacts with water to make carbonic acid, a process that takes about a minute to achieve equilibrium, and then the fun starts.
That formation of carbonic acid triggers a cascading set of reactions called the “carbonate pH stat” that produces some free hydrogen ions (protons), bicarbonate ions, and an exchange between bicarbonate ions and carbonate ions. These relatively quick reactions enable the oceans to absorb 10 times more CO2 than pure water could without them.
Normal seawater is alkaline, which means it has a relatively low concentration of hydrogen ions, so normally the balance of the reactions is tilted in favor of carbonate production. But as more and more CO2 is absorbed, the proportion of hydrogen ions (pH) increases, tilting the reactions away from carbonate (depicted by the tilted balance in the figure above) and generating more bicarbonate. That imbalance is “ocean acidification.” For more detail see this post.
The pH balance can be restored by adding more carbonate, but that’s where we hit a snag.
You will see that the CO2 side of the balance is quick, but on the other side of the balance the processes that replenish carbonate are much, much slower.
Since the atmosphere is in contact with the surface of the ocean (not the deep ocean), it is this smaller reservoir of carbon that is most immediately affected. It increases in acidity and reduces its carbonate levels.
The surface ocean layer is slowly replaced by water from the deep ocean, but that is a process that takes about a thousand years. Carbonate from the land washes in from rivers as a result of rock weathering – but that process is even slower at replenishing carbonate. The result is that for continued large CO2 emissions on a timescale of centuries, the surface ocean bears the brunt and gets ever more depleted in carbonate, and becomes increasingly acidic. Many shell-building marine organisms need water that is highly saturated in carbonate for them to be able to make their shells. As acidification increases, and carbonate saturation decreases, it becomes harder for those organisms to make calcium carbonate shells as their biochemistry has to fight against ocean chemistry increasingly tilted towards dissolving shells, not making them.
As CO2 emissions continue at high rates, it creates a domino effect of ocean chemistry and climate:
All these act in concert to saturate the surface ocean with CO2 and so exacerbate the buildup of CO2 in the atmosphere, accelerating global warming.
The world’s oceans do eventually respond to neutralize excess CO2 and restore balance to the climate – but on a timeframe that will bring little relief for upwards of 30 generations!
Over centuries to millennia, as the oceans mix, the carbonate-depleted surface waters intrude into the realm of the deep, and the surface ocean is replaced by formerly deep ocean waters, which now take up some of the excess CO2 from the atmosphere. As deep water carbonate saturation declines, the depth at which calcium carbonate dissolves (“calcite compensation depth”) rises, causing carbonate sediments and shells below it to dissolve slowly – over millennia - returning carbonate to the seawater in an attempt to restore the carbonate balance. As the surface carbonate levels recover so does the oceans’ ability to process CO2, reducing atmospheric CO2 levels and unwinding the vicious spiral outlined in the bullet points above.
The ocean’s heat absorption capacity, however, works in the opposite direction over this timeframe! To begin with the surface ocean is very efficient at absorbing heat from the atmosphere, as we see today. Over decades to centuries after emissions cease, the surface ocean takes up less and less heat as its temperature approaches equilibrium with the atmospheric temperature. As more heat is taken up by the deep ocean this too eventually reduces its rate of heat uptake, so that after a millennium or more the oceans and the atmosphere reach a thermal balance, stabilizing warmer atmospheric temperatures in proportion to the amount of carbon emitted to the atmosphere (ignoring any feedbacks such as methane release which may add to and sustain the carbon emissions).
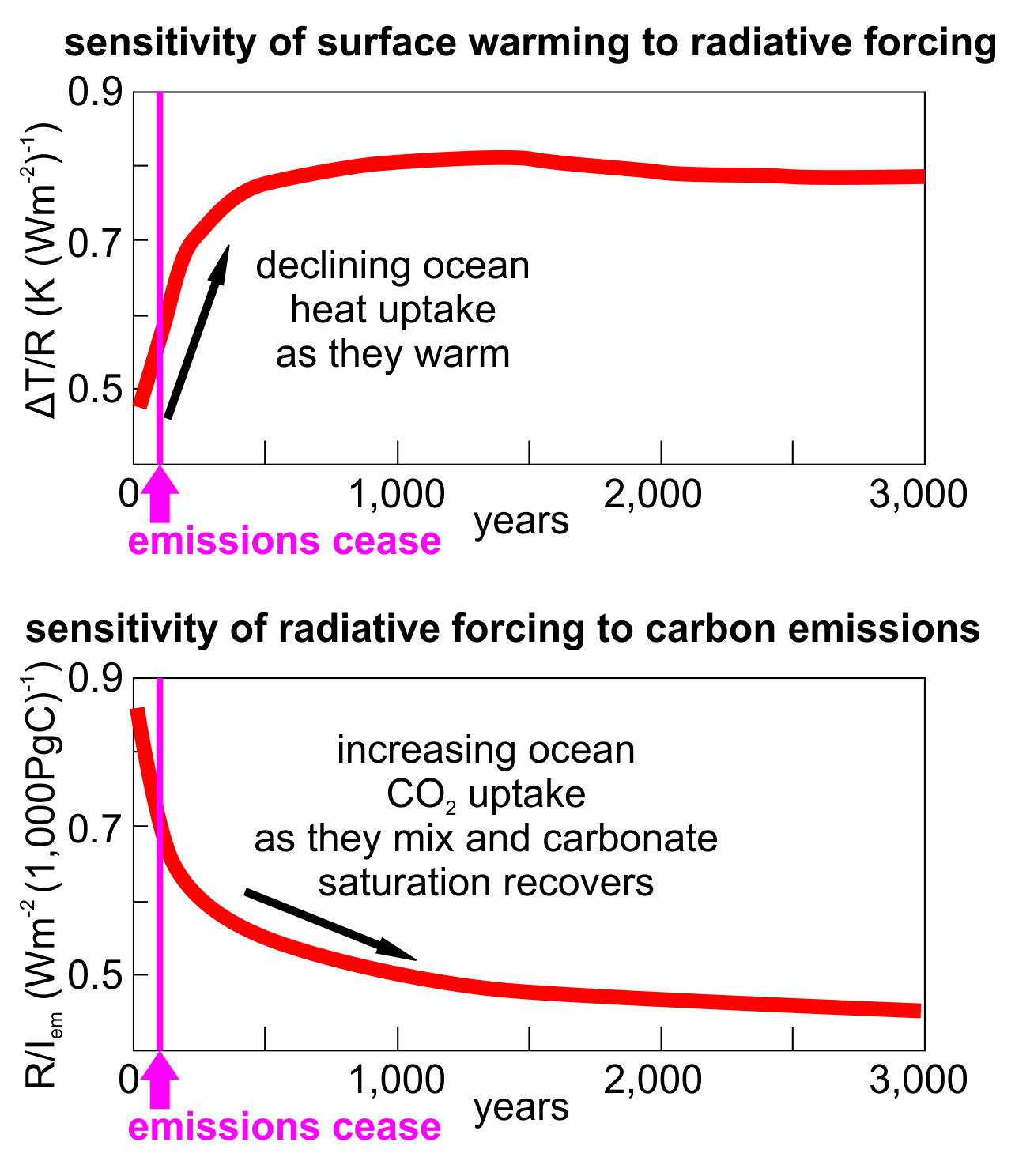 Figure 2: Heat and carbon uptake response to carbon emissions over 3 millennia. Upper graph shows how declining heat uptake by the oceans leaves the climate more responsive to radiative forcing by CO2 in the atmosphere (Y axis units are change in surface temperature per unit of radiative forcing). Lower graph shows how atmospheric CO2 diminishes slowly as the oceans mix and absorb it, gradually reducing radiative forcing (Y axis units are change in radiative forcing per 1,000 Gt emitted carbon). The 2 effects tend to cancel out because even as the oceans remove CO2, the extra heat absorbed by the oceans leaves the climate more sensitive to the CO2 remaining in the atmosphere. Redrawn and simplified from Goodwin et al 2015.
Figure 2: Heat and carbon uptake response to carbon emissions over 3 millennia. Upper graph shows how declining heat uptake by the oceans leaves the climate more responsive to radiative forcing by CO2 in the atmosphere (Y axis units are change in surface temperature per unit of radiative forcing). Lower graph shows how atmospheric CO2 diminishes slowly as the oceans mix and absorb it, gradually reducing radiative forcing (Y axis units are change in radiative forcing per 1,000 Gt emitted carbon). The 2 effects tend to cancel out because even as the oceans remove CO2, the extra heat absorbed by the oceans leaves the climate more sensitive to the CO2 remaining in the atmosphere. Redrawn and simplified from Goodwin et al 2015.
Around 10,000 years or more after emissions cease, the carbonate balance is restored, ending the acidification event as the climate and oceans reach a new steady state when the rate of carbonate added to the oceans by weathering is in balance with the rate of carbonate locked away in sediments and with the CO2 levels of the atmosphere. This makes the ocean carbonate chemistry more favorable for shelly life, even at warmer global temperatures and higher atmospheric CO2 levels.
Over hundreds of thousands of years, rock weathering (which is accelerated a little by warmer temperatures) absorbs atmospheric CO2 and delivers it in the form of carbonate into the ocean where it is locked away as sediments. So over this timeframe the climate will gradually tend to cool again until a new equilibrium is achieved where the rock weathering rate is in balance with the global background volcanic CO2 production rate, with the ocean carbonate pH chemistry linking the two.
It is clear, therefore, that CO2 emission rates faster than the ocean mixing time can overwhelm the surface ocean’s ability to process CO2, and will result in ocean acidification and abrupt global warming as CO2 builds in the atmosphere. In other words, if our emissions continue as they have been then we can expect the surface ocean to be overwhelmed and go through the domino effect outlined above, from now to the lifetime of our grandkids and beyond.
The environmental catastrophe at the end of the Permian period triggered by massive greenhouse gas emissions and acid rain from the Siberian Traps LIP wiped out more than 90% of species on Earth. Recent research has identified 2 pulses of extinction, and although the first extinction pulse may not have involved ocean acidification, widespread ocean acidification was involved in the second pulse 251.88 million years ago. This second pulse saw a sharp rise in atmospheric CO2 levels, a huge 15°C (27°F) jump in global temperatures, and a decrease in pH of 0.7 coinciding with the extinction of many shell-making marine creatures. The ocean acidification lasted about 10,000 years, in line with the time required to replenish ocean carbonate levels.
Scientists estimate that the rate of carbon emissions in the Permian crisis, which came close to extinguishing complex life on Earth, was comparable to current emissions, even if the magnitude and probably the duration of the Permian emissions was much larger.
The end-Triassic was a similar time of mass extinction, triggered by colossal eruptions at the Atlantic margins of both Americas, Europe and Africa. This event appears to have involved 5 intense pulses of volcanic CO2 release, each lasting perhaps 1,000 years or possibly as little as 400 years. Each pulse doubled or tripled atmospheric levels, causing ocean acidification about 0.15 pH units and a drop in reef carbonate production by 15-30%, with levels recovering somewhat in between pulses. The cumulative effect was the loss of some 70% of species on Earth.
Other Large Igneous Provinces had similar effects – the Deccan Traps LIP at the end-Cretaceous, the Karoo-Ferrar LIP during the Toarcian age, and so on. But some LIPs did not trigger environmental catastrophes. Why?
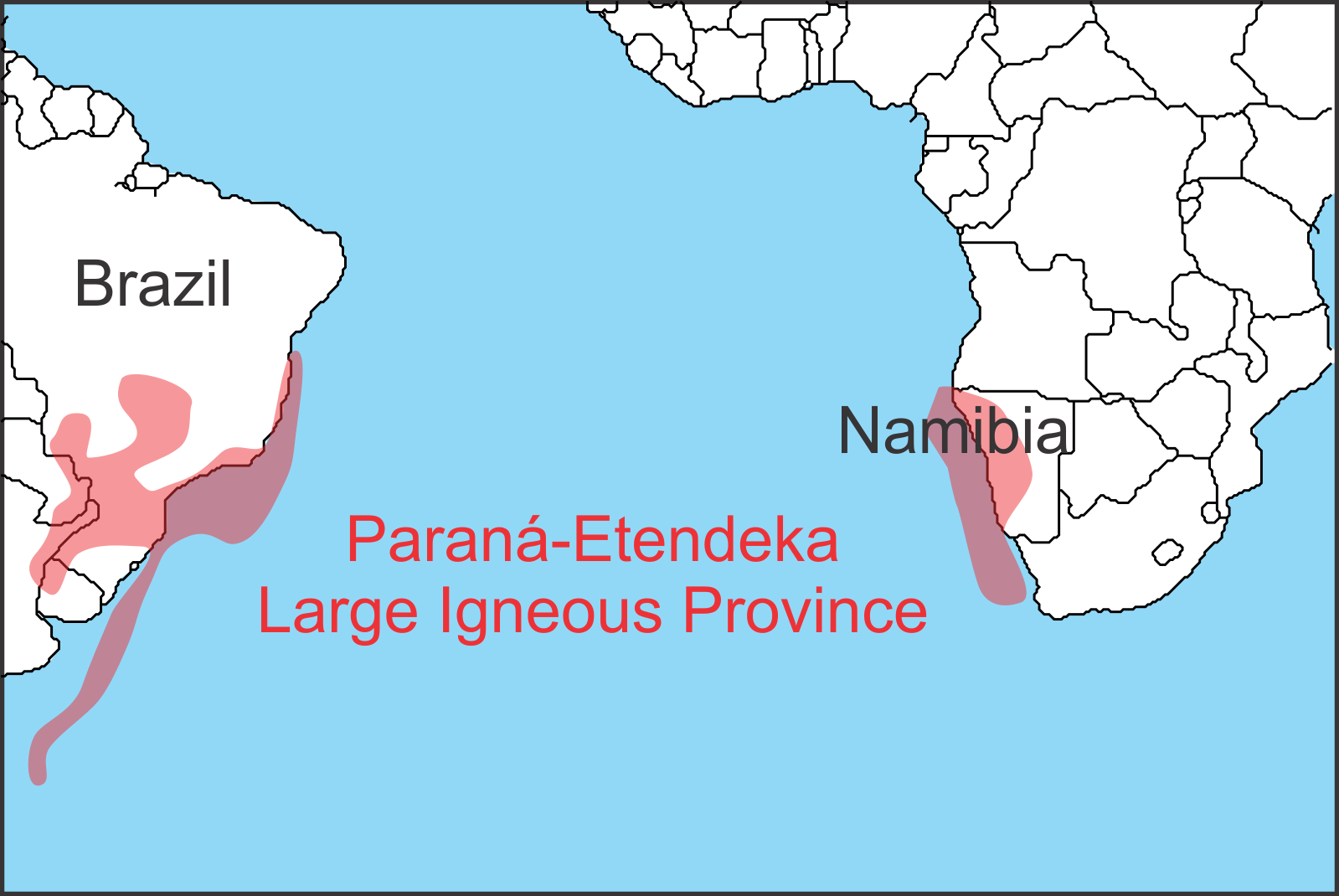
Figure 3: Location of the Paraná-Etendeka LIP. Once a single province it is now separated by the Atlantic Ocean. Based on Bryan and Ferrari 2013.
The Paraná-Etendeka LIP, which erupted 135 million years ago, is one of those relatively benign LIPs despite being a similar size to the destructive LIPs mentioned above.
To figure out why, researchers traipsed across the parched hinterland of Namibia drilling 737 rock samples and hammering off a further 149 pieces of volcanic rock, to analyze the fossil traces of Earth’s magnetic field within them. These samples yielded the direction to Earth’s magnetic pole at the time of their eruption, allowing them to be correlated with published dates for magnetic polarity reversals. This revealed that Earth’s magnetic field reversed 16 times while the Paraná-Etendeka LIP was erupting over a timeframe of 4 to 5 million years – far longer than recent estimates of the duration of other LIPs such as the Deccan Traps (~740,000 years). More importantly, the scientists found no evidence for the kind of intense pulses of eruption that occurred at the end-Triassic and that have also been noted for other LIPs like the Siberian Traps LIP and the Deccan Traps LIP.
The researchers concluded that the Paraná-Etendeka LIP was a slow-burn phenomenon that emitted CO2 slow enough for the oceans' natural compensation mechanisms to neutralize them harmlessly, and therefore it did not trigger a climate shift or environmental crisis.
Emission rates matter. Current human emissions are at a rate comparable to those in Earth’s past that triggered powerful global warming and ocean acidification associated with mass extinctions. If, however, our greenhouse gas emissions were as slow as the Paraná-Etendeka LIP, they would be sustainable and safely processed by the world’s oceans, without triggering dangerous climate change.
Thanks to Pat Hackett, David Kirtley and Andy Skuce for suggestions
Ocean Buffer Chemistry video lecture, one of an excellent series of plain English lectures by Prof David Archer of University of Chicago.
Archer, D. (2010). The global carbon cycle. Princeton University Press.
Pilson, M. E. (2012). An Introduction to the Chemistry of the Sea. Cambridge University Press.
Posted by howardlee on Thursday, 27 August, 2015
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |