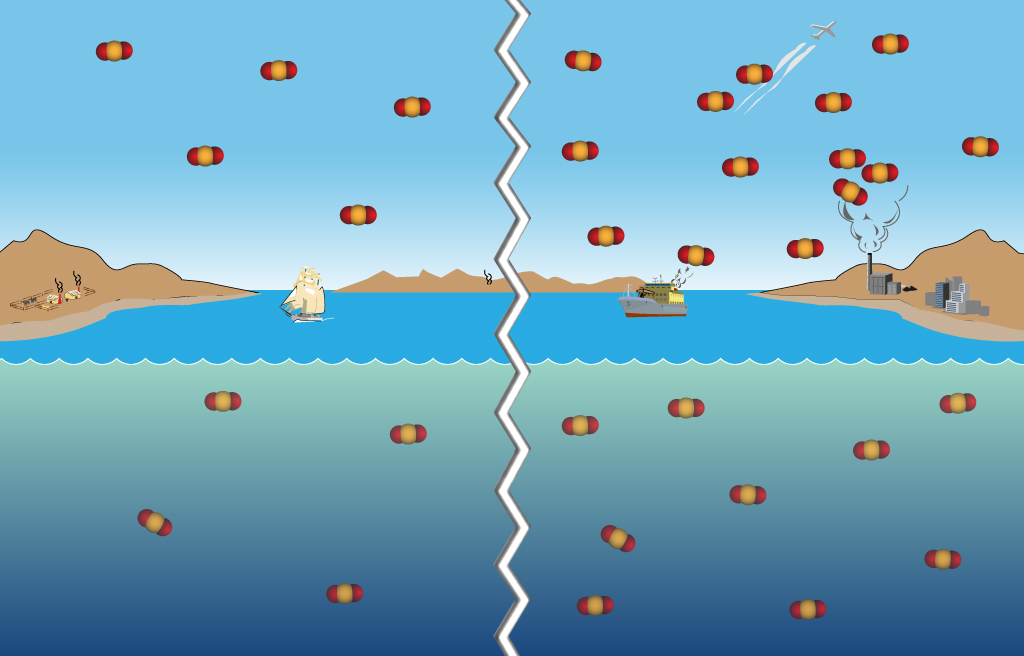
 Figure 1. CO2 added to the atmosphere is absorbed by the oceans due to Henry's law. More CO2 added since pre-industrial times leads to more CO2 absorbed by the oceans.
Figure 1. CO2 added to the atmosphere is absorbed by the oceans due to Henry's law. More CO2 added since pre-industrial times leads to more CO2 absorbed by the oceans.We occasionally receive excellent questions and/or comments by email or via our contact form and have then usually corresponded with the emailer directly. But, some of the questions and answers deserve a broader audience, so we decided to highlight some of them in a new series of blog posts. This is the first one of those posts and more will follow every once in a while in the future.
We recently received an email from Jeffrey Middlebrook who asked about the dynamics of CO2 transport from the atmosphere to the oceans:
...as atmospheric water vapor increases with CO2-driven atmospheric warming, there will be more CO2 capture by the increased water vapor (yielding more carbonic acid) which will transfer more CO2 to the oceans, thereby decreasing the effects of atmospheric CO2 as a greenhouse gas, and with greater precipitation due to more atmospheric water vapor more atmospheric heat will be transferred to the oceans and terrestrial landscapes. It seems plausible that the increases in atmospheric water vapor due to increases in atmospheric CO2 might just produce a strong negative feedback.
About 46% of human emissions of CO2 stay in the atmosphere, while ~26% makes its way to the oceans, and ~28% is used by plants. Our emissions of CO2 may be good for plants (at least for now) but the additional CO2 in the oceans is leading to climate change's "evil twin": ocean acidification. (For much more about ocean acidification see our series: OA is not OK.)
 Figure 1. CO2 added to the atmosphere is absorbed by the oceans due to Henry's law. More CO2 added since pre-industrial times leads to more CO2 absorbed by the oceans.
Figure 1. CO2 added to the atmosphere is absorbed by the oceans due to Henry's law. More CO2 added since pre-industrial times leads to more CO2 absorbed by the oceans.
Think of how large the world oceans are, and the vast ocean surface in contact with the equally vast atmosphere. The oceans primarily absorb CO2 directly from the atmosphere because of the differences in partial pressure across the ocean's "cool skin" layer. If there is more CO2 in a parcel of air than there is in a parcel of water, then the water absorbs more CO2 until there is an equilibrium between the two (Henry's law, see Figure 1). Temperature also plays a role since warmer water holds less CO2 than cooler water.
Another consequence of climate change is the increased capacity of warmer air to "hold" more water vapor. Jeffrey's comment suggests that as more water vapor condenses and forms precipitation more CO2 will be absorbed in precipitation and eventually enter the oceans by this route. Henry's law still operates between the atmosphere and suspended precipitation so there is a bit of CO2 absorption. But the volume of precipitation, say in a massive thunderstorm, is much smaller than the volume of the ocean surface, so this is a minimal effect. The vast majority of CO2 in the oceans gets there directly from the atmosphere via Henry's law.
Absorption of CO2 by the ocean is constrained by the slow, over-turning circulation of ocean waters.
With the oceans absorbing nearly a quarter of our CO2 emissions, will they work to lessen climate change's impacts in the future? Jeffrey alludes to this in his sentence, "It seems plausible..."— more water vapor "scrubbing" more CO2 out of the atmosphere and into the oceans, leaving less and less of an enhanced greenhouse effect in the atmosphere, leading to less and less warming. Unfortunately this scenario takes a very long time--about a thousand years--to play out (see video). Remember that the transport of CO2 from the atmosphere takes place at the ocean's surface which saturates the upper layer of the ocean with CO2. The deeper layers of the ocean are essentially cut off from this exchange. Slow-moving ocean circulation eventually brings fresh, deep water to the surface where it can absorb CO2 from the atmosphere, but this process is too slow to keep up with our emissions.
All the more reason to decrease our emissions of CO2 by switching from fossil fuels to more sustainable energy systems.
Thanks to Daniel Bailey, Gunnar Schade and Glenn Tamblyn for help in answering Jeffrey's email. And to jg for his excellent graphics and video.
Posted by David Kirtley on Tuesday, 6 December, 2016
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |