
In the climate change debate, it appears to be agreed by everyone that excess CO2 will at least have the direct benefit of increasing photosynthesis, and subsequently growth rate and yield, in virtually any plant species: A common remark is that industrial greenhouse owners will raise CO2 levels far higher than normal in order to increase the yield of their crops, so therefore increasing atmospheric levels should show similar benefits. Unfortunately, a review of the literature shows that this belief is a drastic oversimplification of a topic of study that has rapidly evolved in recent years.
The first and most obvious retort to this argument is that plants require more than just CO2 to live. Owners of industrial greenhouses who purchase excess CO2 also invest considerable effort in keeping their plants at optimum growing conditions, particularly with respect to temperature and moisture. As CO2 continues to change the global climate, both of these variables are subject to change in an unfavorable way for a certain species in a certain region (Lobell et al. 2008, Luo 2009, Zhao and Running 2010, Challinor et al. 2010, Lobell et al. 2011). More and more it is becoming clear that in many cases, the negatives of drought and heat stress may cancel out any benefits of increased CO2 predicted by even the most optimistic study.
But there is a more subtle point to be made here. The majority of scientific studies on enhanced CO2 to date have been performed in just these types of enclosed greenhouses, or even worse, individual growth chambers. Only recently have researchers begun to pull away from these controlled settings and turn their attention to outdoor experiments. Known as Free-Air CO2 Enrichment or “FACE”, these studies observe natural or agricultural plants in a typical outdoor setting while exposing them to a controlled release of CO2, which is continuously monitored in order to maintain whichever ambient concentration is of interest for the study (see Figure 1).

Figure 1 - Example FACE study in Wisconsin, USA with multiple CO2 injection plots; courtesy of David F Karnosky, obtained from Los Alamos National Laboratory.
FACE studies are therefore superior to greenhouse studies in their ability to predict how natural plants should respond to enhanced CO2 in the real world; unfortunately, the results of these studies are not nearly as promising as those of greenhouse studies, with final yield values averaging around 50% less in the free-air studies compared to greenhouse studies (Leaky et al. 2009, Long et al. 2006, Ainsworth 2005, Morgan et al. 2005). Reasons for this are numerous, but it is suspected that in a greenhouse, the isolation of individual plants, constrained root growth, restricted pest access, lack of buffer zones, and unrealistic atmospheric interactions all contribute to artificially boost growth and yield under enhanced CO2.
Photosynthesis comes in a few different flavors, two of which are C3 and C4. Together C3 and C4 photosynthesis make up almost all of modern agriculture, with wheat and rice being examples of C3 crops while corn and sugarcane are C4. The distinction deals mainly with the specific enzyme that is used to collect CO2 for the process of photosynthesis, with C3 directly relying on the enzyme RuBisCO. C4 plants also use RuBisCO, but unlike C3 plants, they first collect CO2 with the enzyme PEP-carboxylase in the mesophyll cell prior to pumping it to RuBisCO (see Figure 2).
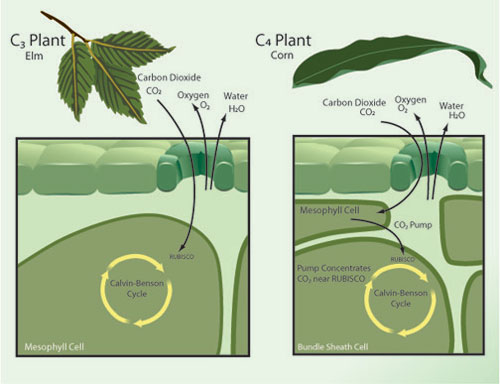
Figure 2 - A simplified diagram contrasting C3 vs. C4 plant photosynthesis. From Nature Magazine.
The relevance of this distinction to excess CO2 is that PEP-carboxylase has no natural affinity for oxygen, whereas RuBisCO does. RuBisCO will just as readily collect oxygen (which is useless) as it will CO2, and so increasing the ratio of CO2/O2 in the atmosphere increases the efficiency of C3 plants; the extra step in the C4 process eliminates this effect, since the mesophyll cell already serves to concentrate pure CO2 near RuBisCO. Therefore excess CO2 shows some benefit to C3 plants, but no significant benefit to C4 plants. Cure and Acock 1986 (a greenhouse study) showed excess CO2 gave a 35% photosynthesis boost to rice and a 32% boost to soybeans (both C3 plants), but only a 4% boost to C4 crops. More recently, Leaky et al. 2006 (a FACE study) did not find any statistically significant boost in photosynthesis or yield for corn (a C4 crop) under excess CO2.
Going a bit deeper, it has recently been found that in some C3 plants—such as cotton and many bean species—a further enzyme known as RuBisCO activase is required to convert RuBisCO into its “active” state, the only state in which it can be used for photosynthesis. The downside of this is that the activase enzyme is much more sensitive to high temperatures compared to RuBisCO itself, and also responds poorly to excess CO2: Heat can destroy the structure of the activase enzyme at temperatures as low as 89.6 F, while excess CO2 reduces the abundance of the cellular energy molecule ATP that is critical for RuBisCO activase to function properly (Crafts-Brandner & Salvucci, 2000, Salvucci et al. 2001). This effect may potentially nullify some of the gains expected from excess CO2 in these plants.
Even within a specific type of photosynthesis—indeed, even within a specific species—the positive responses to enhanced CO2 can vary widely. Nutrient availability in particular can greatly affect a plant’s response to excess CO2, with phosphorous and nitrogen being the most critical (Stöcklin and Körner 2002, Norby et al. 2010, Larson et al. 2010). The ability of plants to maintain sufficient nitrogen under excess CO2 conditions is also reduced for reasons not fully understood (Bloom et al. 2010, Taub and Wang 2008).
It has also been found that excess CO2 can make certain agricultural plants less nutritious for human and animal consumption. Zhu 2005, a three-year FACE study, concluded that a 10% decrease in the protein content of rice is expected at 550 ppm, with decreases in iron and zinc contents also found. Similarly, Högy et al. 2009, also a FACE study at 550 ppm, found a 7% drop in protein content for wheat, along with decreased amino acid and iron content. Somewhat ironically, this reduction in nutrient content is partially caused by the very increase in growth rates that CO2 encourages in C3 plants, since rapid growth leaves less time for nutrient accumulation.
Increased CO2 has been shown to lead to lower production of certain chemical defense mechanisms in soybeans, making them more vulnerable to pest attack and diseases (Zavala et al. 2008 and Eastburn et al. 2010). Other studies (e.g. Peñuelas and Estiarte 1999) have shown production of phenolics and tannins to increase under enhanced CO2 in some species, as well as many alkaloids (Ziska et al. 2005), all of which may have potential consequences on the health of primary consumers. The decreased nutritional value in combination with increased tannin and phenolic production has been linked to decreased growth rate and conversion efficiency of some herbivores, as well as an increase in their relative demand and consumption of plants (Stiling and Cornelissen 2007).
Furthermore, many “cyanogenic” species—plants which naturally produce cyanide, and which include 60% of all known plant species—have been found to increase their cyanide production in an enhanced CO2 world. This may have a benefit to the plants who use cyanide to inhibit overconsumption by pests and animals, but it may in turn reduce their safety as a food supply for both humans and animals (Gleadow et al., 2009a and Gleadow et al. 2009b).
Competing plant species have also been shown to drastically alter expected benefits from excess CO2: even in the best FACE studies, most research still involves artificial experimental plots consisting of fewer than five plant species, and often only one species is present. It has long been understood that due to increased growth of competitor species, benefits from isolated experiments cannot be scaled up to explain how a plant might respond in a monoculture plot (Navas et al. 1999). The distinction is even greater when comparing the behavior of isolated species to those of mixed plots (Poorter and Navas 2003). The lack of correlation (r2 = 0.00) between biomass enhancement (BER) of isolated plants and that of plants in mixed plots is presented in Figure 3.
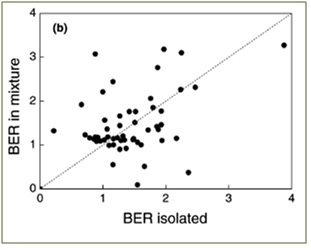
Figure 3 – Isolated vs. mixed biomass enhancement ratios under excess CO2; From Figure 8 of Poorter and Navas 2003
That some plant species may benefit more fully and/or rapidly from excess CO2 also introduces the possibility that the abundance of certain species in an ecosystem will increase more than that of others, potentially forcing the transformation from one type of ecosystem to another (Poorter and Navas 2003). There is also some evidence suggesting that invasive species and many “weeds” may show relatively higher responses to elevated CO2 (Ziska and George 2004), and become more resistant to conventional herbicides (Ziska et al. 2004, Ziska and Teasdale 2000).
There is some evidence that interacting bacterial communities, particularly in the roots, will be affected through elevated CO2, leading to mixed results on overall plant health. Mutualistic fungal root communities (known as ‘mycorrhizae') are typically shown to increase under excess CO2, which facilitate nutrient transport to the roots (Treseder 2004), although infections of pathogenic species such as Fusarium (the agent of the disease known as ‘crown rot’) have been shown to become more severe under excess CO2 as well (Melloy et al. 2010).
It has long been known that stomata (the pores through which plants take in CO2 and exhale oxygen and water) tend to be narrower and stay closed longer under enhanced CO2. This effect is often cited as a benefit in that it increases water efficiency in drought situations.
But there is another key piece to reduced stomatal conductance, considering that 90% of a plant’s water use is actually for cooling of the leaves and nothing more: heat from the sun is absorbed by the water in the leaf, then carried out as vapor in the form of latent heat. So while it is true that the plant may retain water better under enhanced CO2, doing so may cause it to retain more heat. This can potentially carry a plant to less optimal temperature ranges (Ball et al. 1988 and Idso et al. 1993). An image present in Long et al. 2006 (Figure 4) shows this effect quite clearly; while a 1.4 C increase is probably not enough to cause significant damage in most cases, global warming will only serve to exacerbate the effect. It is also of note that the study above represented a well-watered situation, and so during a drought condition the temperature increase would be even higher.
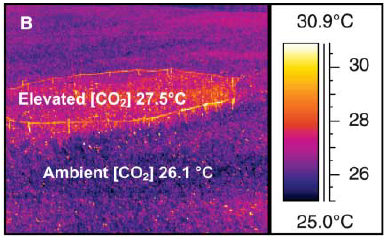
Figure 4 - Increase in local temperature under enhanced CO2 due to reduced evapotranspiration. From Long et al. 2006
On the cold end, it has been found that for seedlings of some species of evergreen trees, excess CO2 can increase the ice formation temperature on the leaves, thereby increasing their sensitivity to frost damage (Roden et al. 1998).
CO2 is not the only atmospheric gas that is on the rise: concentrations of ground-level ozone (O3) are expected to rise 23% by 2050 due to continuing anthropogenic emissions of precursor gases like methane and nitrous oxides. In addition, Monson et al. 1991 found that natural plant emissions of volatile organic compounds (another group of O3 precursors) increase under excess CO2 in many plant species, thereby introducing the potential that local O3 concentrations around plant communities may rise even higher than the baseline atmospheric level.
O3 has long been known to be toxic to plants: Morgan et al. 2006 found a 20% reduction of soybean yield in a FACE study of 23% excess O3. Similarly, Ainsworth 2008 showed a 14% decrease in rice yield at 62 ppb O3, and Feng et al. 2008 (a meta-analysis of 53 peer-reviewed studies) found on average a 18% decrease in wheat yield at 43 ppb O3. Ozone also appears to reduce the structural integrity of plants as well as make them more vulnerable to certain insect pest varieties such as aphids (Warrington 1988).
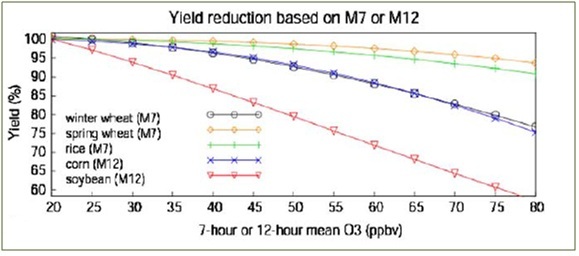
Figure 5 - Yield reduction for several crop species under excess ozone. From Wang and Mauzeral 2004
With respect to this effect, excess CO2 may actually prove beneficial in that it causes a narrowing of leaf stomata, thereby reducing the quantity of ozone that can enter the more sensitive internal tissues. Needless to say, the combined effect of excess CO2 and excess O3 is complex, and as it has only recently been given attention it is an area that requires much further research.
A specific plant’s response to excess CO2 is sensitive to a variety of factors, including but not limited to: age, genetic variations, functional types, time of year, atmospheric composition, competing plants, disease and pest opportunities, moisture content, nutrient availability, temperature, and sunlight availability. The continued increase of CO2 will represent a powerful forcing agent for a wide variety of changes critical to the success of many plants, affecting natural ecosystems and with large implications for global food production. The global increase of CO2 is thus a grand biological experiment, with countless complications that make the net effect of this increase very difficult to predict with any appreciable level of detail.
NOTE: This post is also the Advanced rebuttal to "CO2 is plant food". And a hearty welcome to Dawei to the ranks of Skeptical Science authors
Posted by Dawei on Wednesday, 27 April, 2011
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |