
Dr. Ove Hoegh-Guldberg is a Professor of Marine Studies and Director of the Global Change Institute at the University of Queensland, and one of the foremost experts on the Great Barrier Reef (GBR). This is the second in a three part series from Dr. Hoegh-Guldberg on the state of the GBR. Part 1 is available here.
Coral reefs like the Great Barrier Reef depend on a narrow set of environmental conditions within which they prosper. At the heart of their biology, is a symbiosis that they form with tiny plant-like organisms known as dinoflagellates (commonly called zooxanthellae). This symbiosis is critical to the survival of corals and coral reefs, making possible the efficient trapping of sunlight by reef-building corals. This allows them a cheap source of energy with which to grow in the sunlit waters along tropical coastlines, and where they often deposit of vast quantities of calcium carbonate (a limestone-like substance), creating the three-dimensional structures that we know of as coral reefs. These magnificent structures build up over time, attracting over one million species which are known to live in and around coral reefs (Reaka-Kudla 1997). This mega-diversity is unrivalled anywhere else in the ocean, and supports industries like tourism and fisheries, and provides food for at least 100 million people worldwide (Hoegh-Guldberg 1999).
Coral reefs have come under increasing pressure from the local effects discussed in Part 1 which arise from the rapidly growing coastal populations worldwide. In the early 1980s, coral reefs all over the world began to exhibit a phenomenon known as mass coral bleaching. Mass coral bleaching occurs when the symbiosis between corals and tiny plant-like organisms known as dinoflagellates breaks down. As a result, corals are deprived of their energy source, leaving them prone to disease and death. Over the past 25 years, mass coral bleaching and mortality events have grown in frequency and scale.

Figure 1. Background image shows and extensively bleached reef near Great Keppel Island on the southern Great Barrier Reef in early 2006. The insert shows a normal looking coral on the left-hand side and a bleached coral on the right hand side. The dinoflagellates symbionts which leave the coral is it bleaches are also shown.
The observation that there are no reports of mass coral bleaching prior to 1980 in the scientific literature has led many to conclude that mass coral bleaching is a new phenomenon associated with the activities of humans. The flipside to this argument is that mass coral bleaching events may have occurred in the past but we've only just begun to notice them. This argument falls down for a number of reasons. Firstly, mass coral bleaching events are hard not to notice. When a reef undergoes mass coral bleaching, there is a brilliant white colour that can be seen from passing satellites. Secondly, these events cover hundreds of square kilometres of territory when they occur, and would be as hard not to notice as a bushfire would be on land. Given that scientists have been intensely studying coral reefs since the 1950s (as verified by the scientific literature which contains thousands of articles on coral reefs prior to 1980), it is inconceivable that observers would have failed to note this massive visual change in the ecosystem that they were studying. Lastly, filmmakers such as Ron and Valerie Taylor who have been filming coral reefs since the 1960s do not have mass coral bleaching images on film.
The Great Barrier Reef has experienced six major bleaching events since they began occurring. These events have been growing in scale and size, culminating in 1998 and 2002 in mass bleaching events which affected over 50% of reefs within the Great Barrier Reef Marine Park (Berkelmans; Oliver 1999; Berkelmans et al. 2004).
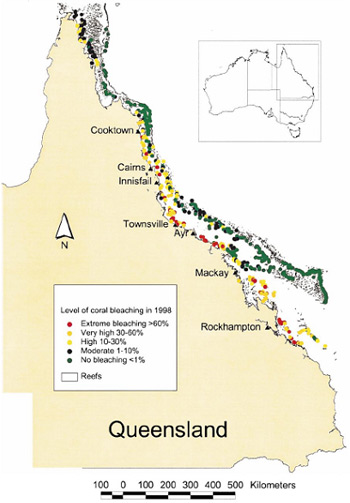
Figure 2. Geographic patterns associated with mass coral bleaching in 1998 (Berkelmans; Oliver 1999).
There is little doubt within the scientific community that elevated sea temperatures are driving the increase in mass coral bleaching and mortality seen on the Great Barrier Reef over the past 25 years. The evidence comes down to 3 key components:
Firstly, experimental studies within aquaria and laboratory settings revealed that corals will bleach when exposed to increases in sea temperature of around 2 to 3°C above their normal maximum temperatures (Glynn; Dcroz 1990; Hoegh-Guldberg; Smith 1989; Jones et al. 1998).
Secondly field study measurements have revealed a close association between small changes in sea temperature above long-term summer maxima and mass coral bleaching events in the field (Berkelmans 2002; Bruno et al. 2001; Dove; Hoegh-Guldberg 2006; Glynn 1983, 1993, 1996; Oliver et al. 2009).
Lastly, satellite detection algorithms based on the laboratory and field evidence have been highly successful in predicting the incidence of mass coral bleaching based on small changes in sea surface temperature. In this case, satellites flown by the National Oceanic and Atmospheric Administration as part of their Coral Reef Watch Program are highly accurate in terms of predicting when and where bleaching will occur.
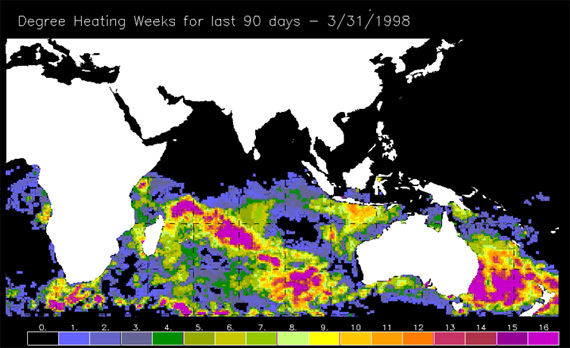
Figure 3. Exposure to elevated heat stress during the lead up to the 1998 bleaching event. Degree heating weeks are calculated by multiplying the size of the sea surface temperature anomaly (relative to the long-term summer average) by the time of exposure. Anything over 4 results in bleaching, while anything above 8 is likely to result in large-scale coral mortality.
Mass coral bleaching represents a threat to coral reefs everywhere. Seawater temperatures are increasing in tropical waters at the rate of 0.1-0.4 degrees Celsius per decade, and will soon be high enough to cause mass coral bleaching every year. Several analyses have been done for coral reefs across the planet (Done et al. 2003; Donner et al. 2005; Hoegh-Guldberg 1999) which have used the known sensitivity of coral reefs and projections of how sea temperature will change over the coming decades. These studies also revealed that coral reefs will bleaching conditions on an annual basis as early as 2030. Given the mortality rates of corals following mass coral bleaching events can be extremely high (over 90% of all corals on a reef dying within a single mass bleaching event) and the fact that it takes at least 15-30 years for a coral reef to recover from a mass mortality event, these trends strongly suggest that coral-dominated ecosystems are very likely to disappear by the middle of the century.
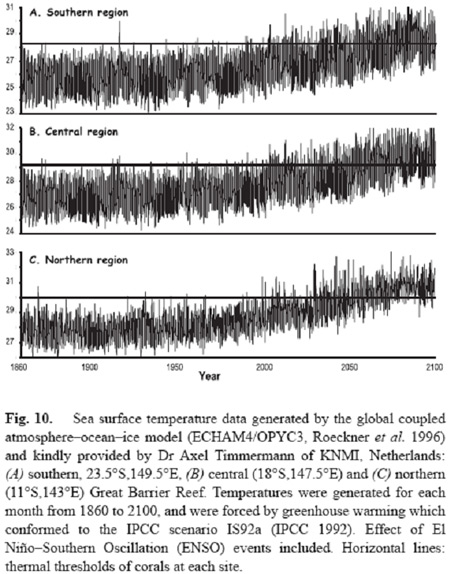
Figure 4. Projected sea temperature projected for the southern, central and northern Great Barrier Reef using a global circulation model (ECHAM4/OPYC3). The horizontal lines in each figure represents the temperature thresholds above which significant bleaching and mortality occur.
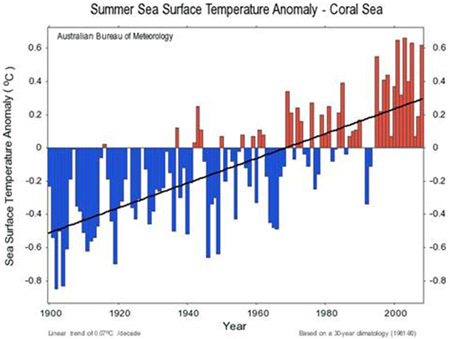
Figure 5. Changes in sea surface temperature over the past 100 years (Australian Bureau of Meteorology).
There has been some debate about whether or not reef-building corals are able to modify their sensitivity to temperature and thereby beat the climate change signal over the time. Evidence cited in favour of this hypothesis has been the observation that corals and some oceans are already experiencing very high sea temperatures. One example are corals growing in the Arabian Gulf, where sea temperatures reach 36°C. The problem with this example is that corals have adapted to their local temperature conditions, but over hundreds if not thousands of years. The ability of an Arabian Gulf coral to migrate and successfully establish a reef in the Indian Ocean over 10 or even 100 years is not feasible given what we know about the migration rate corals.
Another point of discussion has centred on the ability of corals to change their zooxanthellae for varieties that are more tolerant to temperature. This idea that led to the adaptive beaching hypothesis (Buddemeier; Fautin 1993) which proposed that corals bleached deliberately to swap one set of zooxanthellae for more tolerant zooxanthellae. So far, there has not been any compelling evidence that this hypothesis is true. Furthermore, as we have begun to further understand the intricate intracellular symbiosis that corals form with their dinoflagellate zooxanthellae, the more it has become clear that this symbiosis takes an enormous length of time to evolve. That is, to live inside another cell, you need to modify self recognition, integrate biochemically, and undergo a whole series of different changes to ensure that a harmonious endosymbiosis results. The idea that corals could form and use a basis in ecological timeframes of a few years is very unlikely given their long generation times.
Additioanlly, it is extremely unlikely that the thermal tolerance of reef-building corals comes down to that of its zooxanthellae. Heat stress is also likely to be a problem with the host coral, so changing its zooxanthellae is unlikely to solve the problem of rising sea temperatures (Hoegh-Guldberg 1999; Hoegh-Guldberg et al. 2002; Hoegh-Guldberg et al. 2007). This said, some corals do have multiple genetic varieties of zooxanthellae in their tissues. These can be shuffled such that the more thermally tolerant ones can dominate as sea temperatures rise. These separate symbiosis have all evolved with their coral hosts and consequently, provide only limited change in the thermal tolerance of the association as it is challenged by rising sea temperatures. That is, the changes are phenotypic not genetic, and hence do not allow corals to adapt optimally to the novel conditions that are presented by rising sea temperatures.
The conclusion after considering all of the science surrounding the response of coral reefs to changes in sea temperature suggest that coral-dominated reef systems are very likely to disappear from a tropical oceans if we do not reduce carbon dioxide emissions.
In Part 3 of this series, Dr. Hoegh-Guldberg will discuss the impacts of ocean acidification and warming temperatures on coral reefs such as the GBR, and how corals survived past climates with higher atmospheric CO2 concentrations
Berkelmans, R., and J. Oliver, 1999: Large-scale bleaching of corals on the Great Barrier Reef. Coral Reefs, 18, 55-60.
Berkelmans, R., 2002: Time-integrated thermal bleaching thresholds of reefs and their variation on the Great Barrier Reef. Marine ecology progress series, 229, 73-82.
Berkelmans, R., G. De’ath, S. Kininmonth, and W. J. Skirving, 2004: A comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: spatial correlation, patterns, and predictions. Coral Reefs, 23, 74-83.
Bruno, J., C. Siddon, J. Witman, P. Colin, and M. Toscano, 2001: El Nino related coral bleaching in Palau, western Caroline Islands. Coral Reefs, 20, 127-136.
Buddemeier, R., and D. Fautin, 1993: Coral bleaching as an adaptive mechanism. Bioscience, 43, 320-326.
Done, T., P. Whetton, R. Jones, R. Berkelmans, J. Lough, W. Skirving, and S. Wooldridge, 2003: Global Climate Change and Coral Bleaching on the Great Barrier Reef. Final Report to the State of Queensland Greenhouse Taskforce through the Department of Natural Resources and Minings, QDNRM, Brisbane. http://www.nrm.qld.gov.au/science/pdf/barrier_reef_report_1.pdf and http://www.nrm.qld.gov.au/science/pdf/barrier_reef_report_2.pdf, Q. G. Department of Natural Resources, Ed.
Donner, S. D., W. J. Skirving, C. M. Little, M. Oppenheimer, and O. Hoegh-Guldberg, 2005: Global assessment of coral bleaching and required rates of adaptation under climate change. Glob Change Biol, 11, 2251-2265.
Dove, S. G., and O. Hoegh-Guldberg, 2006: The cell physiology of coral bleaching. Coral Reefs & Climate Change: Science and Management, 1-18.
Glynn, P. W., 1983: Extensive Bleaching and Death of Reef Corals on the Pacific Coast of Panama. Environmental Conservation, 10, 149-154.
Glynn, P. W., and L. Dcroz, 1990: Experimental-Evidence for High-Temperature Stress as the Cause of El-Nino-Coincident Coral Mortality. Coral Reefs, 8, 181-191.
Glynn, P. W., 1993: Coral reef bleaching: ecological perspectives. Coral Reefs, 12, 1-17.
Glynn, P. W., 1996: Coral reef bleaching: facts, hypotheses and implications.
Hoegh-Guldberg, O., and G. J. Smith, 1989: The effect of sudden changes in temperature, light and salinity on the population density and export of zooxanthellae from the reef corals Stylophora pistillata and Seriatopra hystrix. Journal of Experimental Marine Biology and Ecology, 129, 279-303.
Hoegh-Guldberg, O., 1999: Climate change, coral bleaching and the future of the world's coral reefs. Mar Freshw Res, 50, 839-866.
Hoegh-Guldberg, O., R. J. Jones, S. Ward, and W. K. Loh, 2002: Communication arising. Is coral bleaching really adaptive? Nature, 415, 601-602.
Hoegh-Guldberg, O., and Coauthors, 2007: Coral reefs under rapid climate change and ocean acidification. Science, 318, 1737-1742.
Jones, R., O. Hoegh-Guldberg, A. Larkum, and U. Schreiber, 1998: Temperature induced bleaching of corals begins with impairment to the carbon dioxide fixation mechanism of zooxanthellae. Plant, Cell and Environment, 21, 1219-1230.
Oliver, J. K., R. Berkelmans, and C. M. Eakin, 2009: Coral Bleaching in Space and Time. Coral Bleaching, 21-39.
Reaka-Kudla, M. L., 1997: Global biodiversity of coral reefs: a comparison with rainforests. Biodiversity II: Understanding and Protecting Our Biological Resources, M. L. Reaka-Kudla, and D. E. Wilson, Eds., Joseph Henry Press, 551.
Posted by Ove Hoegh-Guldberg on Thursday, 7 July, 2011
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |