
This is part 2 of 2 summarising out series about ocean acidification. The posts: Introduction, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
During July and August we posted an 18 part series to introduce the basics of ocean acidification chemistry. In this post we summarise posts 11-18. We have distilled each post to the bare minimum (100 words average). Naturally a lot has been lost; please refer to the original posts.
Part 11: Did we do it? Yes we did!
The International Energy Annual reports that globally, 603 billion tons of CO2 were released from 'consumption and flaring of fossil fuels' between 1980 and 2006 giving an expected change of 77 ppm CO2 in the atmosphere. BUT atmospheric CO2 increased by only 43 ppm. 270 billion tons of CO2 is missing.

Part 12: Christmas present
4 parameters describe the marine CO2 system. If you know any 2 you can calculate the other 2.
Total dissolved inorganic carbon (CO2, H2CO3, HCO3–, and CO32–), Total Alkalinity, pH, and CO2 partial pressure. The partial pressure of CO2 in the atmosphere changes seasonally as plants photosynthesize. Since pH is influenced by the partial pressure of CO2, the pH of seawater also shows seasonal fluctuations. Though the rate of change is not even around the world, all the data agree: ocean pH is decreasing.
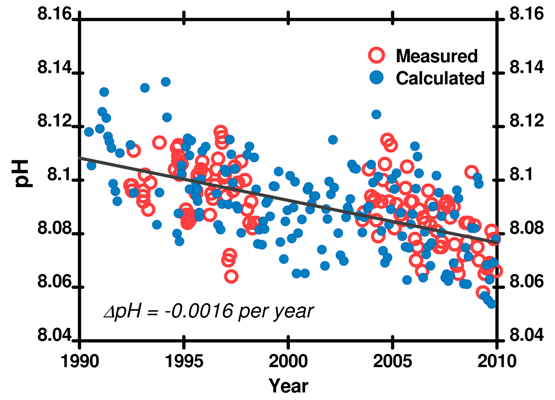 Figure 6. pH recorded at the Hawaii Ocean Time Series (HOTS) station. Data here.
Figure 6. pH recorded at the Hawaii Ocean Time Series (HOTS) station. Data here.
Part 13: Polymorphs: The son of Poseidon
Biological calcium carbonate comes in two main forms, or polymorphs: aragonite and calcite.
Aragonite is found in pteropods (small free swimming snails) and corals. Calcite is found coccolithophores and foraminifera. Molluscs can use either or both polymorphs.
![]()
Part 14: Going down
Photosynthesis in the ocean is confined to the upper 200 m or so where sunlight reaches. Below that depth falling organic matter gets eaten by bacteria (producing CO2). Making CO2 produces H3O+ via Eq. 7 & 8. Dissolution of calcium carbonate via Eq. 4 increases the pH slightly (by removing this acid).

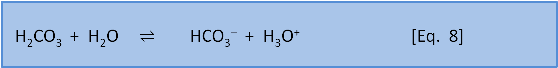
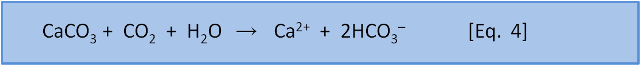
As depth increases, the pH does not recover to the surface value because there is more respiration (producing acid) than shell dissolution (consuming acid). Thus, total dissolved carbon increases with depth (due to respiration) but carbonate decreases with depth because it is a function of pH which decreases with depth.
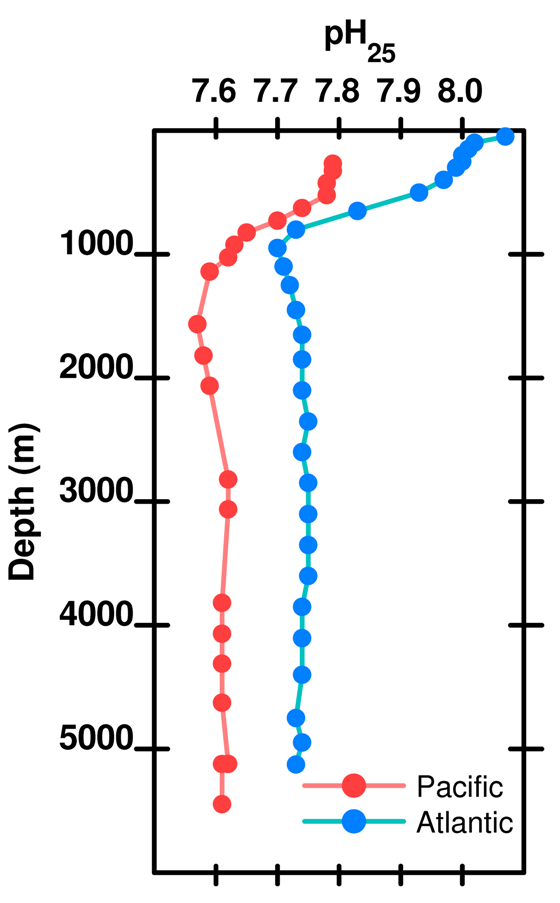 Figure 13. pH (at 25 deg C) as a function of depth in the Atlantic and Pacific oceans. Data .
Figure 13. pH (at 25 deg C) as a function of depth in the Atlantic and Pacific oceans. Data .
Part 15: No accounting for taste
The common ion effect describes the way the dissolution of a salt is inhibited if the solvent already contains an ion in common with the salt (e.g. the solubility of CaCO3 decreases in water containing lots of Ca2+ ions). Similarly, dissolution is enhanced if the concentration of one of the ions, say carbonate, is low (e.g. the dissolution of CaCO3 increases in water if CO32- has been removed by an external factor).
Part 16: Omega

If Ω < 1 the solution is undersaturated and dissolution occurs. If Ω > 1 the solution is supersaturated and dissolution does not occur. For a line at Ω = 1, the depth at which this line crosses the actual Ω gives the depth below which aragonite and calcite dissolve. This is the saturation depth. A poetic image used is to compare Ω to a mountain snow line.
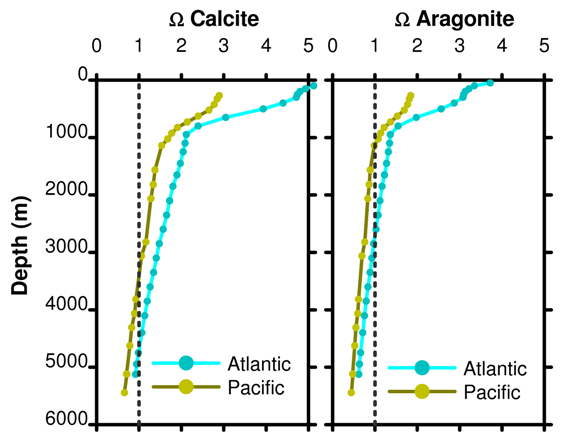
Figure 15. Depth profile of Ω for calcite and aragonite as a function of ocean basin.
Adding CO2 to the surface ocean will cause overall carbon to increase, but also causes the relative fraction of carbonate to decrease. In turn this decreases Ω so the profiles in Figure 15 to move to the left and the saturation horizon moves towards the surface.

Part 17: Pumping currents
Two processes move CO2 from the air-sea boundary into deep waters:
1. Biological pump: Photosynthesis transforms the CO2 into big organic particles that sink rapidly. As the particles fall to the bottom, they get eaten by bacteria, producing CO2 in deep waters.
2. Ocean circulation: The high volume but slow (100s-1000s of years) circulation called the thermohaline ocean circulation swaps surface water with deep water. Added CO2 remains in surface waters until the slow ocean circulation turns the surface waters over. This means the surface ocean is undergoing acidification to a much greater extent than the deep ocean.
Part 18 : Been this way before
We know CO2 levels have been high in the past. What makes this time different?
1. We are outside normal CO2 levels
During an ice age, CO2 is about 180 ppm; during an interglacial CO2 is about 280 ppm. Atmospheric CO2 is currently 394 ppm - 100 ppm above the normal recent maximum.
2. Changes are happening faster than normal.
At the end of an ice age, the 10oC temperature change and the 100 ppm CO2 change occur over 10,000-15,000 years. CO2 is currently increasing at >2 ppm per year – over 100 times faster than the glacial-interglacial cycle.
The ocean carbonate buffer system will not restore the ocean to a preindustrial state. Instead the ocean, and the climate of the Earth as a whole, will converge towards a new stable state. We still don't know precisely what that state will be.
That's all folks. An index with a 2 line description of each post should now greet you when you click the "OA not OK" button at the top left. The booklet will be available in a few days and will be announced in a very brief post.
Written by Doug Mackie, Christina McGraw, and Keith Hunter. This post is the final summary of a series about ocean acidification. Other posts: Introduction, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
Posted by Doug Mackie on Thursday, 25 August, 2011
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |