OA not OK part 14: Going down
Posted on 6 August 2011 by Doug Mackie
This post is number 14 in a series about ocean acidification. Other posts: Introduction, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
Welcome to the 14th post in our series about ocean acidification. In previous posts we have talked about the carbon chemistry of calcium carbonate but we haven't really discussed the main processes that shift and transform carbon in the ocean: photosynthesis and respiration.
Seaweeds like kelp are pretty, but they are not significant in terms of total photosynthesis. Most photosynthesis in the oceans is by the single celled phytoplankton like diatoms and coccolithophores.
Photosynthesis in the ocean is confined to the upper 200 m or so where sunlight reaches. Below that depth life is powered solely by respiration – i.e. eating (or 'burning') organic matter. Back in post 9 in post we noted that: falling organic matter – fish poo and the like – gets eaten by bacteria (producing CO2) as it falls to the bottom.
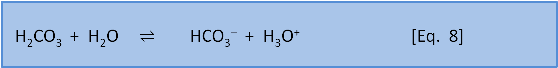
That is, respiration in the deep ocean produces CO2. We know enough about the equilibria in Equations 7-9 from post 5 to say that making CO2 pushes Eq. 7 to the right, making H2CO3 (carbonic acid).

In turn the carbonic acid produces H3O+ via Eq. 8.
Thus, we predict that the deeper you go the lower the pH (i.e. the more H3O+). And, this is just what happens for the first kilometre or so. But below 1 km, we begin to see that the pH does not continue to drop. In fact pH increases a little between 1 and 2 km depth and then more or less stabilises. What is going on?
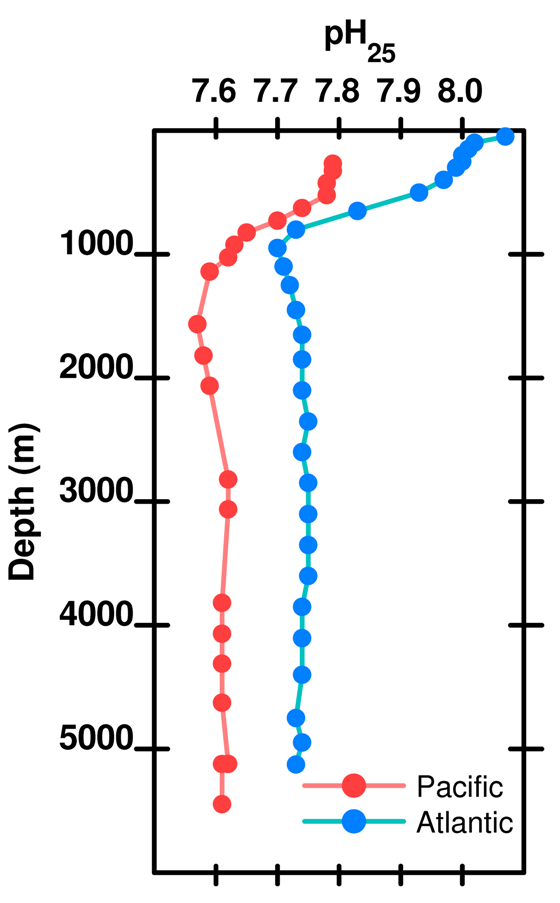
Figure 13. pH (at 25 deg C) as a function of depth in the Atlantic and Pacific oceans. Data are for representative stations from the World Ocean Circulation Experiment . (Data here). Note that there are several scales for pH and several ways of expressing a pH value. Values for pH measured at 25 deg C and surface pressure differ somewhat from the in situ pH at the ambient pressure and temperature.
The pH in the deep Atlantic is around 7.7-7.8, while for the Pacific at depth the pH is around 7.6-7.7. In post 5 we said that by 2100 the pH of the ocean might approach 7.8 and that this was a cause for concern. But, here we have a deep ocean pH of that (or even less) already. If ocean acidification is so bad shouldn't this low pH have some sort of effect?
It does. The low pH water, which was made more acidic by the release of CO2 during respiration, dissolves, or weathers, the sinking calcium carbonate shells of organisms like foraminifera and coccolithophores.
As we saw in post 6, weathering or dissolution of calcium carbonate consumes CO2 (effectively an acid as it readily reacts via Equation 7 to make carbonic acid). That is, dissolution of calcium carbonate increases the pH slightly (by removing an acid). As depth increases, the pH does not recover to the surface value and this tells us that there must be more respiration (producing acid) than shell dissolution (consuming acid). This makes sense because everything in the deep ocean respires but not everything produces a shell that can be dissolved. Note that temperature, salinity and pressure also have a lesser effect on all these equilibrium, but we will ignore them to focus on the impact of CO2.
If we return to the speciation plot (Figure 3 from post 8), enlarged below, we can see that as pH decreases with depth, the amount of carbonic acid increases but the amount of carbonate decreases. (Bicarbonate increases slightly as it approaches the 'hump' in the profile).
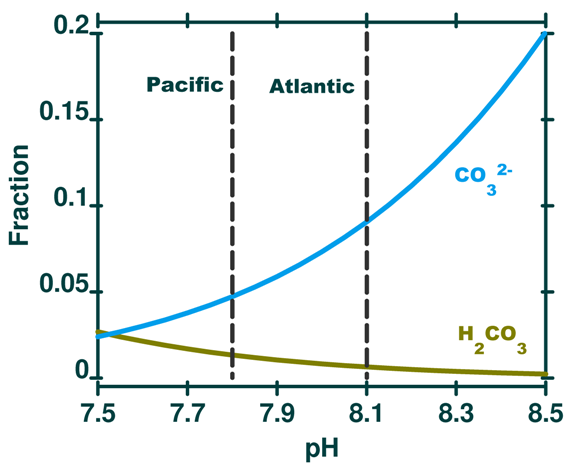
Figure 14. Enlarged portion of Figure 3 from post 8, a speciation plot for the carbonic acid system in seawater. Shown here for the range of realistic pH in seawater. Typical pH for surface Pacific and surface Atlantic noted for comparison by vertical dashed lines. As pH decreases carbonate decreases and carbonic acid increases (bicarbonate also increases over this pH range but is off the scale of this plot - fraction = 0.80 at pH 8.5 to 0.95 at pH 7.5). See the full version in post 8. (The booklet of these posts will include the equations and step by step instructions to draw your own version of Figure 3).
Thus, even though total dissolved carbon increases with depth (due mainly to respiration) the amount of carbonate decreases with depth because it is a function of pH which decreases with depth.
As an aside for the incurably curious: The reason that the Pacific and Atlantic are different is a result of the thermohaline ocean circulation. The deep water circulation starts in the North Atlantic Ocean, where atmospheric cooling near Greenland and Scandinavia generates a downward-moving deep water current. This current flows south through to the Antarctic polar regions where further cold water is added by the same atmospheric cooling process. From there, the current flows into the Indian and Pacific Ocean.
This ocean current sets up a conveyor belt for chemical materials generated by the biological pump. Thus, as biological debris containing both organic tissue and CaCO3 that will soon be dissolved sinks into deep water, it hitches a ride on a current system flowing in the general direction Atlantic to Pacific. As a result, the products of dissolution build up at the end of the conveyor, in the North Pacific Ocean.
Going down: pH isn't the only thing that has changed since the 1980's. In the next post we are building arches.
Written by Doug Mackie, Christina McGraw, and Keith Hunter . This post is number 14 in a series about ocean acidification. Other posts: Introduction, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.































 Arguments
Arguments

























 0
0  0
0 second summary post
second summary post







Comments