Is the airborne fraction of anthropogenic CO2 emissions increasing?
Posted on 31 December 2009 by John Cook
The 'airborne fraction' refers to the amount of human CO2 emissions remaining in the atmosphere. Approximately 43% of our CO2 emissions stay in the atmosphere with the rest being absorbed by carbon sinks. But is the airborne fraction increasing? A paper published in November 2009 found no statistically significant trend (Knorr 2009). Anthony Watts labeled this result the "Bombshell from Bristol" - A potentially devastating result for anthropogenic global warming. Was it such a shock? The 2007 IPCC verdict on the airborne fraction was "There is yet no statistically significant trend in the CO2 growth rate since 1958 .... This 'airborne fraction' has shown little variation over this period." (IPCC AR4) I'm not sure the move from "not much happening" "to "still not much happening" warrants the label "bombshell".
The airborne fraction is calculated from the rate of human CO2 emissions and changes in atmospheric CO2 concentration. The global increase in atmospheric CO2 has been directly measured since 1959 and can be calculated from ice cores for earlier periods. Primarily, CO2 emissions come from fossil fuel combustion with a lesser contribution from land use changes. Fossil fuel combustion is calculated from international energy statistics. CO2 emissions from land-use changes are more difficult to estimate and come with greater uncertainty. Land use emissions are estimated using deforestation and other land-use data, fire observations from space and carbon cycle modeling.
There have been several recent studies determining the airborne fraction. Trends in the sources and sinks of carbon dioxide (Le Quere 2009) examines the airborne fraction from 1959 to 2008. This period was chosen as we have directly measured atmospheric CO2 levels over this time. Fossil fuel emissions rose steadily in recent decades, contributing 8.7 ± 0.5 gigatonnes of carbon in 2008. This is 41% greater than fossil fuel emissions in 1990. CO2 emissions from land use was estimated at 1.2 ± 0.4 gigatonnes of carbon in 2008. Note the proportionally higher uncertainty compared to fossil fuel emissions.
Over this period, an average of 43% of each year's CO2 emissions remained in the atmosphere although there is much year-to-year variability. The noise in the airborne fraction was reduced by removing the variability associated with El Nino Southern Oscillation (ENSO) and volcanic activity. They found the airborne fraction increased by 3 ± 2% per decade. This is a slightly increasing trend although only barely statistically significant .
Knorr 2009 extends this analysis back to 1850 by combining direct CO2 measurements from Mauna Loa and the South Pole with CO2 data derived from Antarctic ice cores. This enabled Knorr to compare CO2 emissions to atmospheric CO2 levels for the past 150 years.
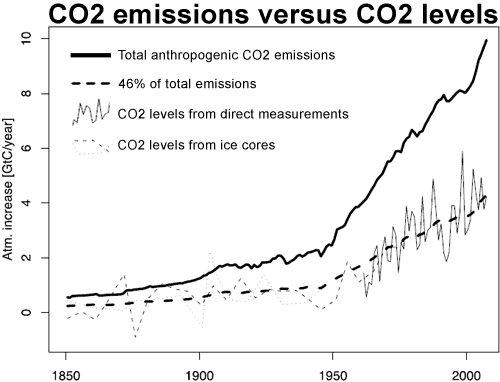
Figure 1: Observed increase atmospheric CO2 derived from direct measurements, taking the average of Mauna Loa (Hawaii) and the South Pole (thin solid line) and two ice cores: Law Dome (dashed thin line) and Siple (thin dotted line). This is compared to total anthropogenic emissions (thick solid line) and 46% of total emissions (thick dashed line). (Knorr 2009)
Knorr finds that since 1850, the airborne fraction has eemained relatively constant. When CO2emissions were low, the amount of CO2absorbed by natural carbon sinks was correspondingly low. As human CO2 emissions sharply increased in the 20th Century, the amount absorbed by nature increased correspondingly. The airborne fraction remained level at around 43%. The trend since 1850 is found to be 0.7 ± 1.4% per decade.
There are several differences in methodology between Knorr 2009 and Le Quere 2009. Knorr's result does not include the filtering for ENSO and volcanic activity employed by Le Quéré. However, when Knorr does include this filtering in his analysis, he finds a trend of 1.2 ± 0.9% per decade. This is smaller than Le Quere's result but is statistically significant.
Knorr also finds the 150 year trend while Le Quéré looks at the last 50 years. This may be significant. If the airborne fraction is increasing, it is possibly a recent phenomenon due to natural carbon sinks losing their absorption ability after becoming saturated. Several studies have found recent drops in the uptake of CO2 by oceans (Le Quere 2007, Schuster 2007, Park 2008). However, with such a noisy signal, this is one question that will require more data before being more fully resolved.
Lastly, some perspective. There are still areas of uncertainty associated with the carbon cycle. Because of this uncertainty, scientists are currently debating whether the airborne fraction is steady at 43% or slightly Increasing from 43%. Unfortunately, some skeptics use this uncertainty to hold the position that the airborne fraction is closer to 0%.































 Arguments
Arguments























 0
0  0
0






Comments