Does breathing contribute to CO2 buildup in the atmosphere?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
| |||
|
By breathing out, we are simply returning to the air the same CO2 that was there to begin with. |
|||||
Climate Myth...
Breathing contributes to CO2 buildup
"Pollution; none of us are supporting putting substances into the atmosphere or the waterways that might be pollutants, but carbon dioxide is not a pollutant. If Senator Wong was really serious about her science she would stop breathing because you inhale air that's got 385 parts per million carbon dioxide in it and you exhale air with about ten times as much, and that extra carbon comes from what you eat. So that is absolute nonsense." (Ian Plimer)
At a glance
We, and almost all of our relatives in the animal kingdom, are aerobic. That means we all depend on this simplified equation in order to function:
glucose + oxygen → carbon dioxide + water + energy
We breathe in oxygen and that oxidises carbohydrates in our body's cells. That chemical reaction gives us the energy required to perform all the varied tasks we do, from blinking to running a marathon. The products of the process are carbon dioxide and water. While the air we breathe in contains just under 420 ppm CO2, what we breathe out contains 40,000-50,000 ppm CO2, a hundredfold increase due to the simplified equation above.
Because we are breathing constantly, this rapid gas-exchange with our surroundings is also constant and, while each of us live, is perpetual. We are part of the fast carbon cycle that involves the movements of carbon through the living world. Of course, the living world also includes plants. Plants take in carbon dioxide to react in the presence of sunlight with the water in their cells. That, in a nutshell, is photosynthesis, the process responsible for the plant-based carbohydrates we eat.
We are vastly outnumbered in terms of carbon biomass by the plant kingdom. Of the estimated nearly 500 billion tonnes of biomass carbon on Earth, the animals account for just 0.4% whilst the plants represent 90%. No wonder that the graphs of measured CO2 levels show an annual fluctuation, forming a symmetrical wobble. The wobble represents the Northern Hemisphere seasons because that's where most of Earth's land masses are found. In the growing season when the plants are busy photosynthesising, CO2 falls, only to rise again in the dormant season. The annual wobble is like the heartbeat of the planet, a regular rhythm along the rising slope that represents our emissions from fossil fuel burning.
Let's imagine a world without fossil fuel-burning. The annual wobble from the seasonal growth and dormancy of plants would be superimposed upon a near-flatline of CO2 levels over human lifetimes. Only occasional events, occurring over tens of thousands to many millions of years, would perturb that near-flatline. That's because there is a second, slow carbon cycle that operates over geological time-scales. In the geologic past, sudden changes in CO2 levels have occurred, primarily due to volcanism on a scale no human, living or dead, has ever witnessed. The fossil record tells us the outcome has never been good.
Fossil fuels are part of the slow carbon cycle. They represent one of several long-term geological reservoirs in which carbon gets locked away. But because we are digging or pumping fossil fuels from the ground and burning them, it is the slow carbon cycle that we are interfering with. No other species has ever intentionally interfered with the slow carbon cycle: this is a first on Planet Earth in its 4.5 billion year long existence. The person quoted in the myth box above is a geologist. He should know better.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
The very first time you learned about carbon dioxide was probably at school, where you were taught that we breathe in oxygen and breathe out carbon dioxide. The process, known as aerobic respiration, is something the vast majority of animals do. In our cells, the following enzyme-controlled reaction is taking place:
C6H12O6+6O2 → 6CO2+6H2O
It's a bit more complicated than that, but the equation is a representative overview. Carbohydrate is oxidised to carbon dioxide and water. The reaction is exogenic - meaning it releases energy at around 3000 Kilojoules per mole of glucose. And while we breathe in air with almost 420 ppm CO2 (2023 figure), it should come as no surprise that the air we breathe out contains 40,000-50,000 ppm (4-5%) CO2, representing a hundredfold increase. That's the product of aerobic respiration.
When confronted with the challenge of reducing our carbon emissions from the burning of fossil fuels, some people angrily proclaim, "why should we bother? Even breathing out creates carbon emissions!"
If someone makes such a statement, they are missing two crucial points. Firstly, our respiration doesn't matter in the big scheme of things. In terms of carbon biomass, we are dwarfed by the plant kingdom. Animals only account for a paltry 0.4% of the estimated near-500 billion tonnes of biomass carbon on Earth. Plants make up 90%.
Through photosynthesis, plants take in carbon dioxide and release oxygen, in a chemical reaction that is essentially the opposite to our aerobic respiration. Plants do perform some respiration, because they need to metabolise as well, but it is outweighed by the photosynthesis. The carbon they collect from the CO2 in the air, converted by photosynthesis into carbohydrates, forms their tissues - roots, stems, leaves, fruit and so on. Such tissues are eaten by all sorts of animals, which in turn are eaten by other animals. We humans are part of this food chain. All the carbon in our body comes either directly or indirectly from plants, which took it out of the air only recently. When we breathe out, all the carbon dioxide we exhale is simply being returned to the air. We are simply giving back the same carbon that was there to begin with. In doing so, we are actively participating in the fast carbon cycle. But our participation is tiny compared to that of plants.
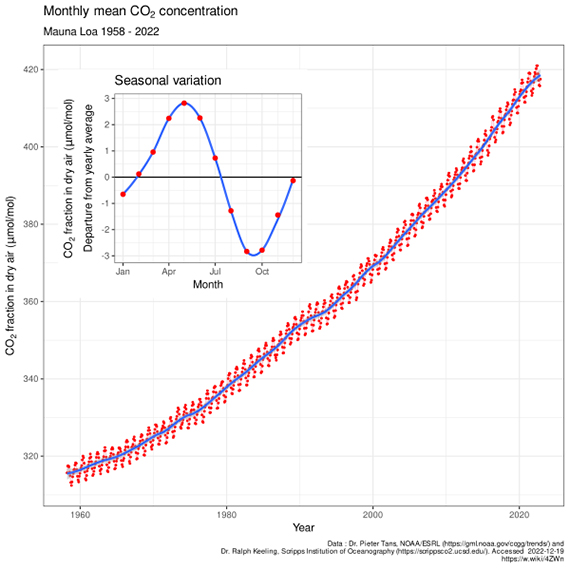
The Keeling Curve (fig. 1) is the graph showing rising CO2 levels as measured at Mauna Loa and other observatories. On it, the plant world's participation in the fast carbon cycle can be seen. Due to photosynthesis, CO2 levels show an annual fluctuation, forming a regular wobble. The downward part of the wobble represents the Northern Hemisphere growing season. Since that's where most of Earth's land is distributed, it's where most of the CO2 drawdown takes place. In the Northern Hemisphere winter, when most plants are dormant, you get the upwards part of the wobble. The wobble, like a planetary heartbeat, is a regular rhythm superimposed upon the rising slope that represents our emissions from fossil fuel burning.
Fig. 1: The Keeling Curve - monthly mean CO2 concentration data (with the occasional volcanic anomaly filtered out), Mauna Loa Observatory, 1958-2022. Inset shows the annual 'wiggle' caused by seasonal plant-growth and dieback in the Northern Hemisphere. Image licensed under the Creative Commons Attribution-Share Alike 4.0 International licence.
Secondly, fossil fuels are the remnants of the fast carbon cycle, fortuitously preserved at various points along the geological time-line. That burial and preservation locked them out of the fast carbon cycle, putting them into the long-term storage part of the slow carbon cycle. Normally the slow carbon cycle operates over geological timescales. Thus, some of the coal we've mined has been more than 300 million years in storage, belonging, appropriately enough, to the Carboniferous period.
Forget about breath. Our carbon emissions from the slow carbon cycle are a) colossal and b) geologically unique. No other species in Earth history has deliberately disturbed the slow carbon cycle. But it has been disturbed - occasionally - by geological processes. Magma has occasionally cooked coal-deposits, as has been observed in Siberia (fig. 2). That rapid release episode, at the end of the Permian period 250 million years ago, didn't work out well. Biodiversity took a massive hit. It recovered – but the recovery took around ten million years.

Fig. 2: masses of coal caught up in basalt, Siberian Traps Large Igneous Province, from Elkins-Tanton et al. 2020. The rising magma interacted with and thoroughly cooked a major coal-basin, releasing a colossal amount of fossil carbon over a few thousand years. The result was catastrophic with the largest mass-extinction of the entire fossil record. Photo: Scott Simper, courtesy of Lindy Elkins-Tanton.
Weathering, plate tectonics, deformation and metamorphism of rocks have all affected CO2 levels - over millions of years. And that's the point. We are doing to our atmosphere, in a few centuries, what most geological processes could only accomplish over millions of years. Through fossil fuel burning, we are performing a unique, vast and uncontrolled experiment with our home planet – the only one we have.
The animation below was published by Dr. Patrick T. Brown (Carnegie Institution for Science, Stanford University) in September 2018, to explain how human respiration fits in to the overall process.
Last updated on 3 December 2023 by John Mason. View Archives































 Arguments
Arguments





























Factor in all the fuel and oxygen spent in agriculture, storage and transport. Then, calculate how deep each of our carbon sink is on average. The more local our food source, such as our back yards, the deeper your carbon sink. Can we dig the treasure of honest living on the beautiful Earth?
So is James Lovelock wrong when he says in his book The Vanishing Face of Gaia (2009):
@dwdwclare If Lovelock is going to take exhalations as contributing to anthropogenic greenhouse gas emissions then you would need to consider the food we eat as being carbon uptake. Respiration is carbon neutral, the carbon in the food we eat originally came from the atmosphere, so when we breathe it out again, we are just returning it to the atmosphere and it has no net effect on atmospheric CO2 levels.
Taking carbon out of the lithosphere and putting it into the atmosphere does however affect atmospheric CO2 concentrations (and indeed increases the total amount of CO2 circulating through the carbon cycle).
It could be that Lovelock is making a subtle point that requires greater context to be apparent.
@Dikran Marsupial Here is the entire paragraph:
Let me also ask, if respiration is carbon neutral why would cow burps and farts add to the amount of greenhouse gasses in the atmosphere? Please don't for a moment think I'm trying to dispute the veracity of anthropogenic climate change being largely caused by the burning of fossil fuels. I'm just asking because I'm curious.
dwdeclare - the issue with cow burps etc is methane - a potent GHG that would not normally be produced if grass were not eaten by a ruminant. Eventually methane oxidizes to CO2 but while present in the atmosphere it contributes strongly to the greenhouse effect. You will see that greenhouse gas inventories are expressed in terms of CO2e (CO2 equivalents) rather than CO2 though the accounting for methane in this method has some issues. Methane from ruminants is increasing only because the number of ruminants has been increased by intensive farming practices.
I think the article should perhaps also mention that there is another way to track the source of the increase in the CO2 in the atmosphere - carbon isotopes. Fossil fuels have no C14. C13 ratios are also different for different sources.
dwdeclare @22, it becomes clear from the broader context that Lovelock is in fact talking about (primarilly) fossil fuel emissions in the production of food rather than mistakenly considering respiration as a form of net emissions. Because those fossil fuel emissions are for the most part normal industrial emissions, they can for the most part be eliminated by the same processes used to eliminate emissions from the rest of industrial civilization, and Lovelock's pessimism is largely unwarranted.
There are a couple of subtleties involved, however, one of which you draw attention to. Methane has a far higher forcing per unit carbon than does CO2. Therefore, the emission of methane by cows in the form of burps and farts represents a transformation of CO2 into methane, and a net short term increase in radiative forcing. Short term because after about 15 years (from memory), the methane in tha atmosphere has transformed back to CO2. The same applies to methane released from swamps (natural) or rice fields (anthropogenic).
The second subtley is far more important. Most fertilizer used in modern agriculture is produced from methane plus components in the atmosphere. The essential step is the Haber-Bosch process:
N2 + 3 H2 → 2 NH3 (ΔH = −92.4 kJ·mol−1)
The hydrogen is produced through one of several processes from methane, of which the two step process may be considered representative:
CH4 + H2O ⇌ CO + 3 H2
CO + H2O ⇌ CO2 + H2
Combining these reactions, we see that for each 8 nitrogen atoms in fertilizer, 3 methane atoms are used in its production, and 3 CO2 molecules released to the atmosphere. By mass, that means that for each 28 Kg of Nitrogen produced in fertilizer, 9 Kg of carbon is consumed as methane, or released to the atmosphere as CO2 in addition to that emitted in producing the energy to drive these reactions.
How significant this is can be seen from wikipedia:
As a rule of thumb, if 80% of nitrogen in human tissue originates from the Haber-Bosch process, then without that process the sustainable population will drop by 80%.
Clearly the hydrogen in the Haber Bosch process could be collected by catalyctic processing of water. That would need to be made significantly more economicly efficient, however, to compete with current methods of production. One of the reasons we should rapidly convert electricity production to carbon neutral methods is just to allow more time before we need to modify the industrial manufacture of fertilizer, both in terms of emissions, and in terms of the availability of methane.
Ah, Haber and Bosch, the two most influential people you've never heard of!