Two Centuries of Climate Science: part two - Hulburt to Keeling, 1931- 1965
Posted on 2 May 2012 by John Mason
The fact that carbon dioxide is a 'greenhouse gas' - a gas that prevents a certain amount of heat radiation escaping back to space and thus maintains a generally warm climate on Earth, goes back to an idea that was first conceived, though not specifically with respect to CO2, nearly 200 years ago. The three-part tale of how this important physical property, its role in the geological past and understanding how it may affect our future, covers about two centuries of enquiry, discovery, innovation and problem-solving.
This is Part Two of this series, which also includes Part One and Part Three.
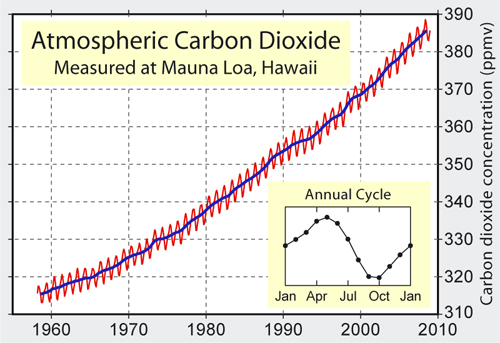
above: atmospheric carbon dioxide levels from the late 1950s onwards. The red wiggles mark out seasonal variations in uptake by plants.
We resume this narrative in 1931, when American physicist E.O Hulburt ran calculations to determine the effect of doubling carbon dioxide once again, and, including the added burden of water vapour, he came up with a figure of around 4°C of warming. He also rebutted Ångström's work and determined that, regardless of convective processes, it was the escape of infra-red radiation to Space (or the hinderance thereof) that was of key importance. The resultant paper appeared in a the journal Physical Review, which tended not to be read by earth and atmospheric scientists and was as a consequence missed by many of them. In any case, it was generally thought that Earth's climate system maintained itself in some natural kind of balance. In retrospect, given the dramatic climate changes that had led to the ice-ages, this was a curious stance to take.
Seven years later, English engineer Guy Callendar, something of an outsider (a steam-engine specialist but with a very keen interest in meteorology), revived the idea, having discovered evidence of a warming temperature trend in the early twentieth century from compilations of temperature records. At long last, the actual levels of carbon dioxide in the atmosphere were revisited: Callendar found they had increased by some 10%, which he suggested may have caused the warming, and he went on to add that over the coming centuries there could be a climate shift to a permanently warmer state.
The reaction at the time was luke-warm: for example, doubt was cast upon the accuracy of carbon dioxide concentration measurements from the nineteenth century. Plus, there were still the old doubts with respect to the original work of Arrhenius: surely the vastness of the oceans would manage to absorb most of that extra gas. Callendar suggested that the top layer of the ocean, that interacts with the atmosphere, would easily become saturated with carbon dioxide and that would affect its ability to absorb more, because, he thought, the rate of mixing of shallow and deep oceanic waters was likely to be very slow. And there was still that old problem of water vapour and carbon dioxide radiation absorption bands overlapping, decreasing the greenhouse properties of the latter gas. Callendar's own calculations, giving a 2°C temperature rise for a carbon dioxide doubling, were slated: one major criticism was that they dealt only with radiation and left out the effects of that other important way in which heat is moved around, convection, despite what Hulburt had already written about that. Again, the prospect of warming causing more cloudiness was raised - something that there were no methods available at the time with which to estimate in terms of amount. All perfectly reasonable objections, simply because there were insufficient data available at the time to clarify matters further.
Such objections, however, led to a renewed drive to unravel parts of the problem, helped by the upsurge in scientific research that accompanied the onset of the Cold War. Atmospheric processes had key implications in military terms, so that it was deemed necessary to understand them as thoroughly as possible, and the properties and behaviour of infra-red radiation came under particular scrutiny, given that if missiles were somehow able to home in on hotspots such as jet exhausts they could seek and destroy such things. The experiments of Ångström, involving air containing various amounts of carbon dioxide in a tube, were found to have been misleading: the problems were down to the relatively low-resolution measuring equipment available at the time. Instead of broad absorption bands, the more precise modern equipment found groups of sharp lines, where absorption would occur, with gaps in between where the infra-red would get through unhindered. Carbon dioxide and water-vapour had their own sets of absorption-lines that did not exactly coincide and it was reaffirmed that water vapour was relatively unimportant in the dryer upper levels of the atmosphere. Now, it was certainly realised that the properties of each layer needed to be taken into account too. Hulburt and Callendar - and indeed Arrhenius - had after all been on the right track, even if aspects of their conclusions were incorrect.
By the mid-1950s, scientists had the huge advantage of the calculating power of computers. This made it possible to dissect each layer of Earth's atmosphere and work out how it might absorb infra-red radiation. Physicist Gilbert Plass undertook the task: firstly his work confirmed that more carbon dioxide would have a warming effect and secondly that doubling levels of that gas would result in a warming of 3-4°C. That, at mid-1950s emissions rates, would be a rise of around 1.1°C per century. Plass wrote that if at the end of the 20th Century the average temperature had continued to rise, it would be "firmly established" that carbon dioxide could cause climate change. But again, the response was luke-warm. The lack of attention to water-vapour and cloudiness led to criticisms of crudeness, and again the matter of the ocean absorbing the extra gas was raised in objection to Plass' suggestion that the extra carbon dioxide would remain in the atmosphere for a thousand years.
The 1950s was the era of nuclear tests. Amongst the fallout from nuclear explosions was carbon 14, an unstable isotope of carbon that has six protons and eight neutrons in the nuclei of its atoms (the most abundant by far, forming 98.9% of all carbon on Earth, is carbon 12 with six protons and six neutrons). Because carbon 14 is unstable, it undergoes radioactive decay, and through this radioactivity it can be tracked as it moves around in the atmosphere. The tracking enabled scientists to establish that within a matter of years any long-lived gases added to the atmosphere are well-mixed throughout all layers, from pole to pole. But carbon 14 also forms high in the upper atmosphere, where cosmic ray bombardment occurs. This is a constant process compared to the one-offs that represent each nuclear explosion, a factor that allowed another test to be made.
Carbon 14 has a short half-life, which is why radiocarbon dating is only used for getting ages for relatively recent things and not ancient stuff like rocks that are millions of years old, amongst which can be counted the fossil fuels. In coal and oil, all the carbon 14 has long since decayed away, so that burning them would only release non-radioactive carbon 12 and the much rarer but stable carbon 13. Burning fossil fuels on a massive scale would therefore add more carbon 12 and 13 to the air relative to carbon 14, regardless of nuclear tests. Chemist Hans Suess put this to the test by examining carbon isotopes in trees. He found that the younger the wood, the more carbon 12 and 13 there was relative to carbon 14. This was the fingerprint of fossil fuel-burning, recorded in the wood.
At the time, the increase was small, reinforcing the idea that the oceans were absorbing much of the added carbon dioxide. However, follow-up research was commenced by Suess, working with Roger Revelle at the Scripps Institution of Oceanography, and by other specialists: all came to a similar conclusion independently of one another, which was that the ocean would typically have claimed any molecule of carbon dioxide emitted within about a decade. However, Revelle, something of a specialist in sea-water chemistry, was aware that the various chemicals present in sea-water have buffering effects that work to keep sea-water at a slightly alkaline state. Revelle suggested that the buffering would place a strict limit on the amount of carbon dioxide the oceans could actually absorb.
This was a critical step in the research. Revelle calculated that, at the emissions-rates of the time (assuming, like most of his predecessors, that these would likely remain constant), an increase of atmospheric carbon dioxide levels of around 40% was possible over the coming centuries. However, as an aside he did note that if emission-rates kept on increasing, the outcome would be different with significant warming in the decades ahead. Importantly, he pointed out that human beings were now carrying out a large-scale geophysical experiment of a kind that could not have happened in the past or be reproduced in the future - an allusion, perhaps, to the growing realisation of the finite, one-off nature of the fossil fuels, being as they are a non-renewable resource over human timescales.
The significance of the limited ability of the oceans to absorb carbon dioxide caught on after a while and was elaborated upon by Swedish meteorologists Bert Bolin and Erik Eriksson, who explained what happens. Basically, although the gas is indeed easily absorbed by sea-water, it is the timescales that matter: mixing of shallow and deep oceanic waters takes place over hundreds to thousands of years but sea-water can de-gas parts of its carbon dioxide payload over much, much shorter periods. Like their predecessors, Bolin and Eriksson ran the calculations regarding possible temperature changes for a doubling of carbon dioxide, but this time assuming emissions would increase and increase yet more on an ever-steepening upward path. They wrote of a 25% increase of atmospheric carbon dioxide by the year 2000. This was far more drastic than anything previously had suggested and Bolin warned that a radical change in climate might occur, a statement echoed by Russian climatologist Mikhael Budyko in 1962.
So, what was happening to the atmosphere's carbon dioxide content? Was it really going up? The only way to find out would be to start monitoring the levels of the gas by accurate measurement, so moves were made to do just that, starting with a network of 15 measuring-stations around Scandanavia. The results were incredibly noisy, but then flaws were found in the methodology. However, in California, researcher Charles David Keeling improved the techniques, to the point where he felt that it might be possible to isolate and remove spurious sources of noise. Revelle and Suess took him on, funding his time and equipment. Locations far from noisy, local manmade and natural carbon dioxide sources were chosen, in places such as Antarctica and atop the Mauna Loa volcano in Hawaii. It should be mentioned here that, at the latter site, the prevailing wind is off the ocean and the fissures that emit gases are almost always downwind: if the wind changes the sudden upticks due to volcanogenic carbon dioxide are so blindingly obvious that they can easily be removed. Anyway, the idea was to establish a baseline concentration and then see what the levels would be in subsequent years. By 1958, Keeling was confident that he had the baseline reliably nailed and two years later he reported that levels were rising, at a rate that might be expected if the oceans were not taking in most of the emissions as detailed above.
Sadly, the Antarctica stations fell victim to a lack of funding. However, the Mauna Loa monitoring station was continued and it continued to find an increase. The measurements also picked-up a well-defined and regular fluctuating cycle corresponding to the growing seasons of plants in the northern hemisphere. There was a decrease in Spring and Summer and an increase in Autumn and Winter marking increased and decreased uptake of carbon dioxide respectively. In the meantime, a steadily increasing understanding of other aspects of the complex carbon-cycle was ongoing. The aim in general was to work out how much of the carbon dioxide resulting from the burning of fossil fuels was ending up in the oceans, vegetation, soils, weathered minerals and so on. Importantly, this multidisciplinary work at last brought together the various branches of science that had previously been working in relative isolation: atmospheric scientists, biologists, geochemists, computer specialists and so on. In 1965, it was announced that "By the year 2000 the increase in atmospheric CO2 ... may be sufficient to produce measurable and perhaps marked changes in climate."
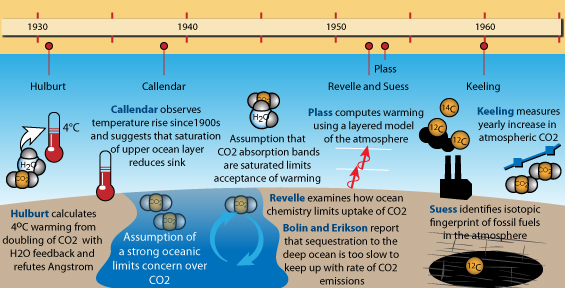
Major discoveries in climate science, 1930-1960. Image by jg
Further Reading
Spencer Weart's The Discovery of Global Warming gives a very detailed account of the history of climate science with a plethora of references - there are many days' worth of in-depth study there for those who want to go beyond the blogosphere!































 Arguments
Arguments
























 0
0  0
0






Comments