Recent Comments
Prev 2018 2019 2020 2021 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 Next
Comments 101251 to 101300:
-
muoncounter at 00:42 AM on 30 December 2010A Positive Outlook For Clouds
#10: " ...latent heat of water which doesn't have an associated temperature change at the surface." I don't understand that mechanism. Evaporation from a surface cools the surface. As that vapor rises it will condense, losing its latent heat to the surrounding atmosphere. Whether or not the resulting clouds live long enough to produce precipitation back to surface is highly variable. As would be the so-called negative feedback due to the albedo of said clouds. Is it therefore correct to say no 'associated temperature change' or just 'no equivalent radiative (SB) temperature'? -
Rob Painting at 00:38 AM on 30 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
Argus @ 43 - Please come back soon and show us December as well From Jeff Masters' Wunderblog here
From Jeff Masters' Wunderblog here
-
Daniel Bailey at 00:36 AM on 30 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
Re: Argus (43) Here's the link. Make sure you re-set the projection to polar before running. December data not yet available. The Yooper -
Rob Painting at 00:30 AM on 30 December 2010The Scientific Guide to Global Warming Skepticism
When CO2 leaves the ocean, does this mean that the ocean becomes less acidic? Yes, generally speaking the tropical regions are more alkaline than more temperate regions, because of the decreased solubility of CO2 in warm water. But remember that we are adding more and more CO2 to the atmosphere all the time, so the partial pressure also goes up, driving more CO2 into the ocean and so increase its' capacity to store CO2. Does warming offset the acidification problem to any extent? Not to any useful degree, as explained above. Ocean warming has its' own set of worrying consequences, but staying on topic, the oceans still have the capacity to absorb considerably more CO2, just depends how much more it we dump into the atmosphere. -
mlyle at 00:24 AM on 30 December 2010A Positive Outlook For Clouds
It doesn't take much of an analysis to show that clouds cannot have a very strong negative feedback. If so, the feedback would have damped out the glacial cycles. Growth to maximum ice sheet size only made about a 20% change in albedo of the earth, holding clouds constant. If there were a strong negative feedback, clouds could easily have compensated. -
muoncounter at 00:18 AM on 30 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
#44: " ...the perfect example of "warmist" cherry-picking!" So now we're even and the cherry-picking can stop. Objectively, the map at #40 should be of concern, whether you happen to temporarily be in an island of blue or not. Even if you take the Spencer graph (Fig 1 in the post) at face value, the lows are all moving higher. So it is no longer relevant to talk about one cold winter as signifying the trend has stopped. If your argument is 'a cold winter means GW has stopped', then you must really panic every year as the days grow shorter. -
Argus at 00:02 AM on 30 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
dhogaza at 00:13 AM on 29 December, 2010, "Meanwhile, the capital of Greenland is forecast to be 24F above the historical average for January on Sunday ..." You (unwittingly?) supply the perfect example of "warmist" cherry-picking! One place in Greenland where the forecast is supposed to be proof of global warming... -
Argus at 23:58 PM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
Daniel Bailey, #40: Please come back soon and show us December as well, and we'll see how that looks! -
Paul Barry at 23:06 PM on 29 December 2010The Scientific Guide to Global Warming Skepticism
Apologies for all the typos above(51). I hit "submit" instead of "preview". I hope it is still intelligible. -
Paul Barry at 23:00 PM on 29 December 2010The Scientific Guide to Global Warming Skepticism
(a) CO2 and the oceans @John (reply@47) The caption says: “Warming causes the oceans to give up more CO2”. In your response you say “...more of our CO2 emissions remain in the atmosphere each year (the airborne fraction)”. To me these sound like very different propositions. One implies CO2 is leaving ocean and going into the air, the other suggests the ocean has reached its capacity and so no long takes up CO2 which therefore remains in the atmosphere. Conceptually that is completely different. Is this even the same phenonemon? Or are you saying that the first proposition is a quite of simplification that is not really accurate? Is this not confusing? @Rob Painting (@50) Thanks for your reply and the graphic. I take it that a positive flux of CO2 in the graph (red) means CO2 is leaving the ocean because it is warming. Negative (blue) means the ocean is taking up more CO2. When CO2 leaves the ocean, does this mean that the ocean becomes less acidic? Does warming offset the acidification problem to any extent? (b) The graph of ocean heat content @John (reply @ 47) You say "if he'd have had access to direct ocean heat measurements down to the abyssal depths, I'm guessing the ocean heat graph would've shown less year to year variability." I agree with you there. I think intuitively the graphs looks wrong. We are told on the one hand that the ocean stores most of the warming that is taking place, and then we get this graph which suggests that this ocean heat content is more variable than the heat content in the atmosphere. I'm still unsure about how reliable Murphy et al's data is given the problems with ocean heat content measurements discussed elsewhere in this site. @Eric (Skeptic) @ 49 "So basically the ocean is storing heat over the long run but the rate of storage can fluctuate based on weather-related ENSO cycles in the Pacific." The problem, Eric, is that the fluctuations in heat content in the ocean suggested by the graph are way out of scale with what one would imagine. ENSO has a large effect on surface temperatures, but its influence on ocean temperatures should be proportionally far less than this graph suggests given its enormous heat capacity. I hope you can see now the problem with the way this particular graph is included in the guide. There are far too many questions about it. You do at least need more information about it perhaps a link to a page on your own site in the reference section explaining what it is AND WHAT IT IS NOT. These things come back to haunt. Thanks for your efforts. We are making progress. -
Ed Davies at 22:44 PM on 29 December 2010Back from the Dead: Lost Open Mind Posts
Bookmarked, many thanks. The whole legal situation with that archive is interesting, though. Firstly, of course, there's the question of copyright: just because a page is available for public reading I don't see that it follows that it's licensed for anybody to come along, take a copy and display it on another site. Secondly, presumably Tamino or his ISP removed the pages in question due to some legal threat so I wonder why that wouldn't apply to the archive as well. And, yes, I'm puzzled by the motivation as well but grateful in this case. -
Alexandre at 22:05 PM on 29 December 2010Back from the Dead: Lost Open Mind Posts
Thanks Daniel, you have helped me before with those links. This Wayback Machine amazes and puzzles me, it must be a huge memory bank. I wonder what was is made for in the first place (apart from helping us retrieving these great long gone webpages for free, of course). -
Dick Veldkamp at 21:45 PM on 29 December 2010The Hitchhiker’s Guide to the AGU Fall Meeting
Mr Cook, I think you are a bit harsh on Greg Craven. From what I gather from the transcription of his talk, his self-assessment is correct: it’s a bit over the top – but not more than that (and frankly, some other talks I watched were rather boring, in spite of the importance of the subject matter. So maybe a “performance” was a welcome change). However let us look past the presentation, at his message. Greg Craven raises an important point: should scientists speak up more in the climate change debate? There are no easy answers, but judging by the deluge of misinformation, and especially the appalling quality of most newspaper reporting on climate change (the notorious “balanced reporting”), I would tend to answer “Yes”. On the other hand, it may be argued that scientists lose authority by engaging in debate. But then again: what good does that authority do us if warnings are not heeded anyway? The way I see it, we are still continuing with business-as-usaul, which is the certain road to disaster (just look around at what is already happening). The sole fact that there was no ice on Greenland the last time CO2-concentration was at 280 ppm should be enough for a radical policy change (yes, I am living in the Netherlands). But nothing is happening. So maybe it is time for more screaming and less polite discourse. -
Alden Griffith at 21:02 PM on 29 December 2010Back from the Dead: Lost Open Mind Posts
Thank you for posting this!!!! -
MarkR at 20:58 PM on 29 December 2010A Positive Outlook For Clouds
#1 Palmer: climate models are unanimous on a positive water vapour feedback (but this also increases the negative lapse rate feedback*), and combined water vapour/albedo bring total global warming to 2-3 C for a doubling of CO2. Dessler, 2008 finds good observational evidence for positive water vapour feedback too... But clouds can have a very, very big effect and, in principle, sufficient to cancel out the water vapour effect. The numbers do appear to 'add up' but models generally disagree and observations can't seem to find it either (discounting Lindzen & Choi which was successfully eviscerated by Trenberth et al IMO) *by increasing the radiative heating on the surface, you encourage more evaporation. Earth no longer has to transfer all of its heat back up radiatively (which is related to temperature through Stefan Boltzmann), but instead more of it goes as latent heat of water which doesn't have an associated temperature change at the surface. -
MarkR at 20:54 PM on 29 December 2010A Positive Outlook For Clouds
#7: Soundoff - I believe this has been calculated by Wolfendale, Sloan & Erlykin but I can't remember where they published this (Prof. Wolfendale told me in person, I can't remember the link) They work on cosmic rays & clouds and there is a negative correlation between cosmic rays and low clouds and a positive one between cosmic rays and medium clouds. Pro cosmic-rays scientists say it's because of cosmic rays, Wolfendale/Sloan/Erlykin found that clouds mostly change before cosmic rays and that much of it could be explained by solar warming raising the clouds (so that some 'low' clouds are now counted as 'medium', hence the correlation). Of course, this is a transient effect and I'm not sure what the overall global, secular effect would be expected to be. -
gpwayne at 19:40 PM on 29 December 2010Back from the Dead: Lost Open Mind Posts
Shouldn't that last Rod Sterling quote say "For civilization to survive, the human race has to become civilized"? -
Daniel Bailey at 19:15 PM on 29 December 2010Back from the Dead: Lost Open Mind Posts
Note: Due to database limitations, the remaining Open Mind post listings are in this comment. Enjoy! Posted on November 9th, 2011Response:[DB]
- Dec 9, 2010 Hide the Incline with the “Rank Trick”
- Dec 13, 2010 Odd Man Out
- Dec 16, 2010 Comparing Temperature Data Sets
- Dec 20, 2010 Open Thread
- Dec 21, 2010 It’s the Trend, Stupid
- Dec 27, 2010 History of Arctic Sea Ice, part 2
- Jan 2, 2011 Hottest Year
- Jan 6, 2011 Sharper Focus
- Jan 14, 2011 Monckton Skewers Truth
- Jan 18, 2011 How to Hide the Decline (from yourself)
- Jan 19, 2011 MLE
- Jan 20, 2011 How Fast is Earth Warming?
- Jan 21, 2011 Phil Jones was Wrong
- Jan 24, 2011 Loaded Questions
- Jan 25, 2011 Milankovitch Cycles
- Jan 27, 2011 Glacial Cycles, part 1
- Jan 27, 2011 Paul Nurse on science vs anti-science
- Jan 28, 2011 Glacial Cycles, part 1b
- Jan 29, 2011 Glacial Cycles, part 2
- Jan 30, 2011 AMO
- Feb 1, 2011 Peer Pressure
- Feb 3, 2011 A challenge to Dr. Roy Spencer
- Feb 3, 2011 Not a Misquote. A Nonquote.
- Feb 8, 2011 Bullseye
- Feb 12, 2011 Ridge Regression
- Feb 12, 2011 Maybe we’re getting through … ?
- Feb 17, 2011 Open Thread
- Feb 19, 2011 The Champion
- Feb 19, 2011 My Hero(ine)
- Feb 20, 2011 Fooled Again
- Feb 22, 2011 Fooled Yet Again
- Feb 24, 2011 While We Fiddle …
- Feb 26, 2011 Mathturbation
- Mar 2, 2011 8,000 years of AMO?
- Mar 3, 2011 Where’s the Global Warming?
- Mar 5, 2011 Snow
- Mar 7, 2011 Mission Failure
- Mar 11, 2011 Dishonor among deniers
- Mar 13, 2011 Eyjafjallajökull
- Mar 21, 2011 Food for Thought
- Mar 31, 2011 So What?
- Apr 1, 2011 Richard Muller Love-Fest
- Apr 1, 2011 Northeast U.S. Snowfall
- Apr 11, 2011 CO2 shame
- Apr 11, 2011 Jerk
- Apr 18, 2011 Cherries Jubilee
- Apr 28, 2011 Hide the Incline
- Apr 29, 2011 Airborne Fraction
- Apr 30, 2011 The China Syndrome
- May 7, 2011 Hell and High Water
- May 8, 2011 Gullible’s Island
- May 9, 2011 Favorite Denier Tricks, or How to Hide the Incline
- May 9, 2011 Ice Out
- May 10, 2011 Five Years
- May 14, 2011 Fake Forcing
- May 17, 2011 Hot … and Wet
- May 20, 2011 Warts and All
- May 21, 2011 Seven Months Ago
- May 25, 2011 Year of the Twister
- May 26, 2011 Math Fun: the Markov Tornado
- May 27, 2011 Markov 2
- May 28, 2011 Methane Update
- June 2, 2011 Circle Jerk
- June 2, 2011 Frankly, Not
- June 9, 2011 Must-Watch Video
- June 13, 2011 Regime Change
- June 14, 2011 Chaos
- June 16, 2011 Open Thread
- June 19, 2011 Volcanic CO2
- June 23, 2011 Sea Ice 3-D
- June 24, 2011 Mike Mann Responds
- June 28, 2011 Skeptics: Real or Fake?
- July 6, 2011 Aligning Station Records
- July 13, 2011 Bob Carter Does his Business
- July 16, 2011 Trend and Noise
- July 20, 2011 Ice Forecast Update
- July 22, 2011 How Not to Analyze Tide Gauge Data
- July 30, 2011 Open Thread
- Aug 1, 2011 Ice Forecast Update Update
- Aug 3, 2011 Cumulative Sums
- Aug 6, 2011 Bag of Hammers II
- Aug 10, 2011 Settled Science
- Aug 11, 2011 Crock
- Aug 12, 2011 Can Bastardi Learn?
- Aug 12, 2011 Learning from Bastardi’s Mistakes
- Aug 20, 2011 Temperature Prediction: the next few months
- Sept 1, 2011 Arctic Sea Ice: Death Spiral Continues
- Sept 4, 2011 Odd Introduction to a New Paper
- Sept 7, 2011 Fred Singer
- Sept 14, 2011 Busy, busy, busy, …
- Sept 15, 2011 Climate Reality
- Sept 16, 2011 Climate Reality Success
- Sept 17, 2011 Cherry Herring
- Sept 17, 2011 Cold Cherries from Joe D’Aleo
- Sept 17, 2011 More Cherry Ice from Joe D’Aleo
- Sept 19, 2011 Merchants of Doubt
- Sept 21, 2011 Fruit Loops
- Sept 21, 2011 God help Texas. Rick Perry won’t.
- Sept 23, 2011 Misalignment
- Sept 24, 2011 How to be a Fake Skeptic
- Sept 25, 2011 Three More Months To Go
- Sept 30, 2011 Unnatural variation
- Oct 4, 2011 Arctic Sea Ice 2011, 2012
- Oct 7, 2011 NCEP
- Oct 8, 2011 Seasons Change
- Oct 9, 2011 Survey Says…
- Oct 14, 2011 Truth or Consequences
- Oct 15, 2011 Opportunity Knocks?
- Oct 15, 2011 A Stitch in Time
- Oct 20, 2011 Berkeley Team Says Global Warming NOT Due to Urban Heating
- Oct 23, 2011 Fake Skeptic Criticism of “Decadal Variations in the Global Atmospheric Land Temperatures”
- Oct 24, 2011 Decadal Variations and AMO, Part I
- Oct 28, 2011 Decadal Variations and AMO — Part II
- Oct 28, 2011 Republican “Science”
- Oct 30, 2011 Judith Curry Opens Mouth, Inserts Foot
- Nov 1, 2011 Questions for Judith Curry
- Nov 4, 2011 Why Not Weighted?
- Nov 5, 2011 Question of the Week
- Nov 5, 2011 The Real Problem with the Global Warming “Debate”
-
gallopingcamel at 17:31 PM on 29 December 2010Comparing all the temperature records
dhogaza (@51), You said: "And, it was a much rarer event than your cold spell, as Russian meteorologists have described it as "unprecedented" - proxy reconstructions going back 1,000 years show nothing at all like the month long heat wave." You may be right, but proxy reconstructions fall far short of what the historical record can tell us. The years 1538 to 1541 were remarkable owing to the extremely hot summers throughout Europe. There were periods of 7 months or more with extreme heat. It was possible to wade across the river Seine in Paris. While London experienced very hot summers over this period, the river Thames froze in 1506, something that has not happened since 1814. Here is a link that you may find interesting: http://booty.org.uk/booty.weather/climate/1500_1599.htm -
Eric (skeptic) at 14:25 PM on 29 December 2010A Positive Outlook For Clouds
rocco, yes, I didn't mean to imply a negative feedback, just a currently negative forcing. The change in that forcing in response to the AGW warming will be the feedback (that is what the paper is trying to figure out). The NRCF does go positive locally and often goes positive at night in large areas of the planet. It could easily go globally-averaged positive for short periods without disaster. The net forcing returns to negative with normal cloud processes that won't be any different in an AGW-warmed world (processes won't change, but frequency, geography, etc will change) Also that forcing is somewhat balanced against latent heat transfer, another reason why positive is not a disaster. -
SoundOff at 14:10 PM on 29 December 2010A Positive Outlook For Clouds
This is a question. Would it be reasonable to suppose that a warmer atmosphere might have stronger convective forces, therefore cloud formation would shift to slightly higher altitudes overall? I’d appreciate your thoughts on this. -
Phila at 13:19 PM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
Nederland: merits the level of hysteria exhibited by some in the field of climate change? Right now, I would have to say no. "Hysteria" isn't very descriptive, I'm afraid. It's also a rather ugly term thanks to its misogynist roots. I suggest that instead of using intellectually empty and emotionally prejudicial terms like this one, you identify the specific responses to AGW to which you object. That way, we can stick to the facts instead of getting bogged down in identity politics.Moderator Response: Indeed, and a better place for Nederland to do that is the thread "It's Not Bad." -
rocco at 12:57 PM on 29 December 2010A Positive Outlook For Clouds
Eric (skeptic): Yes, but that is cloud radiative forcing, not feedback. It is unlikely that the sign of NCRF will change (if it does = absolute disaster) -
actually thoughtful at 12:52 PM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
Regarding focusing on record highs and lows. An individual high or low record tells us nothing more than that weather exists. If you find many, many more record highs than lows, then you could surmise that we are in a warming trend. Both are true. A post this year on Skeptical Science did the research. More alarmingly, we are in a La Nina (cold regime) and a solar minimum and STILL having record warm temperatures. What do you think will happen when we switch back to neutral or El Nino? What do you think will happen when the sun increases in activity? These two accidents (La Nina and solar minimum) are encouraging the do nothing crowd and when those influences are replaced by the normal to active range, people will clamor for action (and ignorant radicals will say it is ALL due to the sun or the El Nino) -
Eric (skeptic) at 11:40 AM on 29 December 2010A Positive Outlook For Clouds
Two radiative components involved are long wave and short wave. The long wave component depends on cloud top temperature compared to the surface temperature ("heat trapping" is more just the suppression of radiational cooling). Add the two components and get the net: negative (cooling) or positive (warming). More on that in 12.2 here https://rams.atmos.colostate.edu/AT712/proofs/ch12PostProofing.pdf The doc above says the global average is currently net negative and has a discussion of how that might change mainly as a result of how circulations like the Hadley might change. -
Daniel Bailey at 11:01 AM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
We have to remain cognizant that the perception of vast sea of humanity is heavily coloured by the weather (their 'tree-blindness" makes them unable to perceive the forest around them). In Argus' case (being from Sweden), his perceptions and hence his opinions are heavily influenced by this: Perhaps a room at the "It's Freaking Cold" thread could be permanently procured for him...
The Yooper
Perhaps a room at the "It's Freaking Cold" thread could be permanently procured for him...
The Yooper
-
villabolo at 10:56 AM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
Nederland, with reference to #35 and "hysteria". I stated at the end of the post that there is enough of a temperature change to disrupt weather systems and adversely effect crops. You have to realize that with the Arctic ice cap in decline and the subsequent exposure of more open seas, we are going to see a dramatic change in weather patterns. What's more, this change could start impacting us within a decade or two. -
Daniel Bailey at 10:51 AM on 29 December 2010Is it safe to double atmospheric Carbon Dioxide over a 200 year period?
Re: GC (107) Thanks for the heads-up on your interesting article. Let me know when you append to it. I will be circumspect. The Yooper -
muoncounter at 10:06 AM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
#35: "... the world is getting warmer and that human activity is the cause--obviously it is." Great! "a rate of warming that merits the level of hysteria exhibited by some in the field of climate change" Can you provide evidence of this 'level of hysteria'? I'm more familiar with deniers becoming hysterical, especially when their claims are refuted. See the existing thread 'Is the IPCC alarmist?' for further discussion. -
villabolo at 09:55 AM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
Argus with reference to your comparison between 'skeptics' and 'warmists' in #19 there is a distinction to be made. 'Skeptics" always cherry pick their dates and places as well as give contradictory responses (They would mention 'Global Cooling' one moment and 'natural' warming the next.). It's the big picture that counts-that cannot be said often enough. Let's take the winter of 2009/2010 which was similar to ours. Just by eyeballing NASA satellite images you could tell that only about 10-15% of the Earth was cooler than normal while the other 85%+ was much hotter. The Arctic Ocean and most of Canada was 5-10 degrees Celsius warmer! The Southern Hemisphere had the hottest year on record. It is with this knowledge that you can then proceed to highlight individual events. Skeptics NEVER take the big picture. -
dhogaza at 09:45 AM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
Looking at your graph in Figure 3, I'd say that the data shows warming at a rate of around 0.15 deg/decade. While that amount of warming will undoubtedly cause many changes for our planet, I'm not sure those changes should be labelled "catastrophic".
Note that this will accelerate because 1. the planet hasn't reached equilibrium thus even if CO2 were held constant as of today we'd continue to see temperatures rise for a long time. 2. we're adding CO2 to the atmosphere at an exponential rate, which will increase the rate of additional warming on top of that in the pipeline. Also understand that a 0.15 deg/decade rise in global temps means a rise at two or three times that rate over north america and eurasia. And lastly, "CAGW" is a denialist term. Climate scientists don't use it. And the mainstream position is that we still have time to limit warming to 2C if we take sufficient action now, though it's becoming increasingly obvious that we won't. The point, though, is that it's not scientists talking about "CAGW" as some sort of horror story foregone conclusion. -
Nederland at 09:22 AM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
You asked what the past 30 years of temperature data really show. Looking at your graph in Figure 3, I'd say that the data shows warming at a rate of around 0.15 deg/decade. While that amount of warming will undoubtedly cause many changes for our planet, I'm not sure those changes should be labelled "catastrophic". Please note that I am not denying that the world is getting warmer and that human activity is the cause--obviously it is. What I am asking is this: does the current evidence suggest a rate of warming that merits the level of hysteria exhibited by some in the field of climate change? Right now, I would have to say no. -
Esop at 09:11 AM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
About cold and warm temp records: 19 countries set national all time warm records in 2010 while only a single one set an all time low national record. The only thing that is relevant when discussing whether AGW is happening or not is the long term trend in average global temperature. 2010 will almost certainly be the warmest on record in most datasets. In case someone missed it, the average global temperature last week (UAH lower troposphere) was the highest on the posted record. As long as the global average is at the highest level recorded, warm local records are a natural and expected result of that, while local cold records are interesting and shows us that increasing the energy imbalance of the system does not cause even warming all over, but can cause local cold snaps as well. That tells us that it is even more crucial to cut greenhouse emissions and reduce the energy imbalance than if warm weather was the only problem, so skeptics highlighting unusual weather when the average is at record high levels would normally not be such a bright idea. However, the MSM love to lap up the disinformation and feed it to the ignorant masses, so the "skeptic" tactics work. -
Esop at 08:52 AM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
#32 (dhogaza): Priceless :) Let us see if the current normal (and above normal temps) in England continue and what Mr. Corbyn will have to say. I would not be surprised if they drop again, though. Something is majorly messed up with the Arctic circulation patterns. -
kdfv at 08:33 AM on 29 December 2010It's freaking cold!
I have just been looking at the NCDC US climate at a glance for November 2010 depature from normal http://www.ncdc.noaa.gov/oa/climate/research/cag3/cag3.html It shows the temperatures generally around 4 to 6 degrees above normal. Yet when I view the US temperature map for the same month accessed from the same page it just shows the majority of states at or below normal? I don't see how the two maps relate. -
LucAstro at 08:31 AM on 29 December 2010A Positive Outlook For Clouds
Thanks for this summary on clouds effects on AGW. -
Rob Painting at 08:16 AM on 29 December 2010The Scientific Guide to Global Warming Skepticism
Paul Barry @ 48 - The example of positive feedback on page 3 has a caption saying “Warming causes oceans to give up more CO2”. Elsewhere we are told that the oceans are getting more acidic - i.e. more dissolved CO2. So which it? Is CO2 in the ocean going up or down or both? Is it different in different places? Is one from the deep sea and the other not? Why is it so paradoxical, if so? The following graphic from NOAA gives an idea of John what is talking about: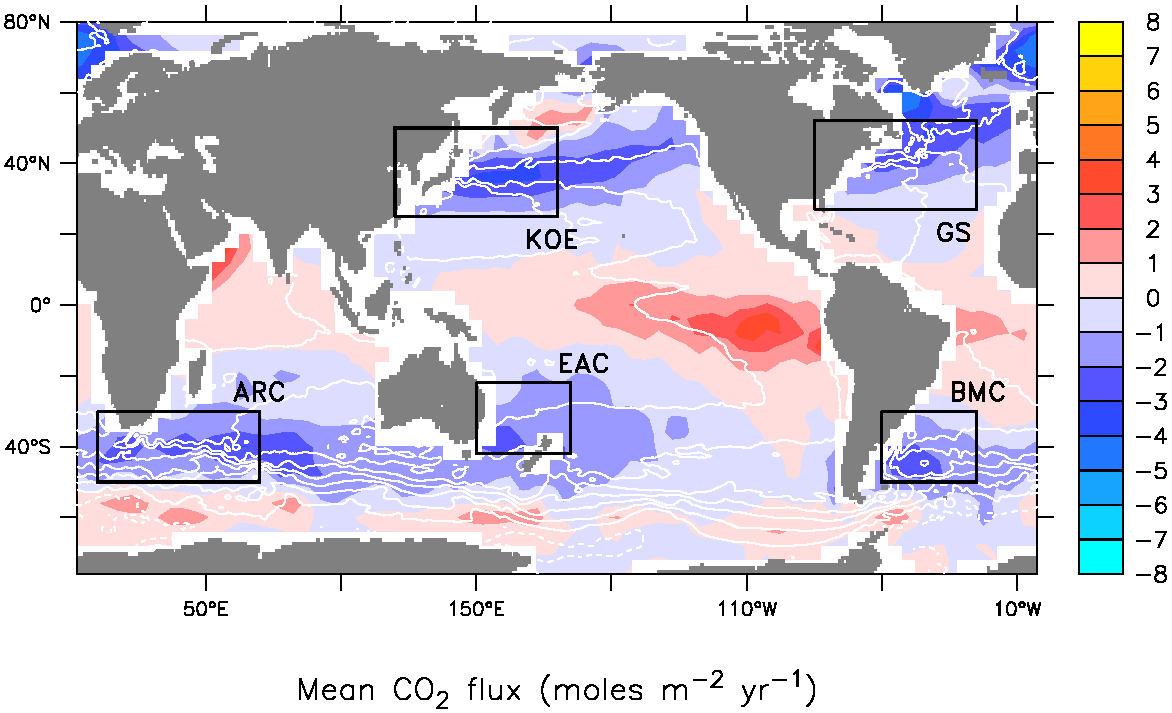 A simplified version is that CO2 is less soluble in the oceans at higher temperatures. You can see the band of CO2 flux to the atmosphere around the tropical regions, where water temperatures are higher, and the absorption of CO2 in cooler waters (Note the obvious warming around Antarctica & flux of CO2 to the atmosphere).
So you can see that both absorption and release of CO2 from the oceans can occur at the same time, it's a matter of temperature, the net effect at the moment is seeing CO2 build up in the ocean, as also evidenced by increasing ocean acidification measurements and declines in carbonate saturation states. So there's no contradiction.
Now as the oceans continue to absorb more heat their ability to store CO2 will diminish, and we'll see the red regions in the graphic start to rise.
A simplified version is that CO2 is less soluble in the oceans at higher temperatures. You can see the band of CO2 flux to the atmosphere around the tropical regions, where water temperatures are higher, and the absorption of CO2 in cooler waters (Note the obvious warming around Antarctica & flux of CO2 to the atmosphere).
So you can see that both absorption and release of CO2 from the oceans can occur at the same time, it's a matter of temperature, the net effect at the moment is seeing CO2 build up in the ocean, as also evidenced by increasing ocean acidification measurements and declines in carbonate saturation states. So there's no contradiction.
Now as the oceans continue to absorb more heat their ability to store CO2 will diminish, and we'll see the red regions in the graphic start to rise.
-
thepoodlebites at 08:15 AM on 29 December 2010It's Pacific Decadal Oscillation
Re: #43 Thanks for the link, I like the title "Open Mind," with the Douglas Adams subtitle. Don't really care for the name-calling but I guess it comes with the territory. The GISS baseline of 1951-1980 is during mostly negative PDO, while the UAH 1980-2000 is during mostly positive PDO. The UAH baseline is warmer than GISS which explains some of the differences. Comparing the 12-month running means using the same 1980-2000 baseline, there doesn't appear to be any significant warming since the 1998 El Nino. But the GISS plot in post #39 seems to show an upward trend continuing through 2008. I think that the GISS plot is misleading, which is unfortunate. Please allow me to review the material more and get back to you. I'm concerned about the integrity of the surface observing sites that GISS et al. uses, possible contamination from urbanization. The satellite data represents better ground-truth, I think. Whenever raw observations are altered (corrected), the data no longer represents ground-truth but are best described an an analysis. -
gallopingcamel at 08:12 AM on 29 December 2010Is it safe to double atmospheric Carbon Dioxide over a 200 year period?
Daniel Bailey (@87), It took a while but I managed to write up my visit to NOAA in Asheville that look place in October. You and "scaddenp" requested a link so here is the first of two parts: http://diggingintheclay.wordpress.com/2010/12/28/dorothy-behind-the-curtain-part-1/#more-1204 By sending you the link I have "outed" myself. How will that work out? -
dhogaza at 07:23 AM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
"Just to be clear you're telling the population of the UK the weather is "dead normal" at the moment?" As of 8:18 PM the temperature in London was 45F. Average high for January is 45F. -
Bibliovermis at 07:10 AM on 29 December 2010Lindzen and Choi find low climate sensitivity
I see the post I was replying has been removed through moderation. The contention was that satellite temperature data showed a decreasing temperature. -
Bibliovermis at 07:07 AM on 29 December 2010Lindzen and Choi find low climate sensitivity
George White (co2isnotevil), That is a wholly false contention. Why did you link to a unreferenced chart? What source data did you use? What atmospheric level? Why did you convert the data to absolute temperature rather than leave it in its native temperature anomaly format? It's interesting that your purported satellite data shows no spike in 1998. Here is the actual satellite temperature data. Source: University of Alabama in Huntsville - lower troposphere satelitte temperature data Moderator Response: The post you are responding to was deleted due to a number of off-topic and inflammatory comments. If you would like to discuss the temperature record, please move the discussion to Are surface temperature records reliable? or Warming Stopped in 1998.
Moderator Response: The post you are responding to was deleted due to a number of off-topic and inflammatory comments. If you would like to discuss the temperature record, please move the discussion to Are surface temperature records reliable? or Warming Stopped in 1998. -
Alexandre at 07:03 AM on 29 December 2010A Positive Outlook For Clouds
Dana, thanks for the post. That figure says roughtly the same thing I've seen on Dr. Archer's lectures (recommended, btw). But I have also heard otherwise, and Nasa's figure on your link has arrows with different proportions. Are these rough behaviours still subject of considerable uncertainty? Or can it already be said for sure that high clouds mainly retain heat whereas low clouds mainly reflect sunlight? -
Albatross at 06:47 AM on 29 December 2010It's Pacific Decadal Oscillation
Re #43: Please read this. And also note the trend in global SATs associated with previous moderate to strong La Nina events (ONI <-1.0. Global SAT anomalies from GISTEMP are for the latter year for each event relative to 1951-1980 baseline: 1949-1950: -0.16 C 1954-1955: -0.10 C 1955-1956: -0.17 C 1964-1965: -0.11 C 1970-1971: -0.10 C 1973-1974: -0.08 C 1975-1976: -0.16 C 1984-1985: +0.04 C 1988-1989: +0.19 C 1998-1999: +0.32 C 1999-2000: +0.33 C 2007-2008: +0.44 C 2010-2011" +0.50 C (?) -
RSVP at 06:39 AM on 29 December 2010Stratospheric Cooling and Tropospheric Warming - Revised
Both of you guys can do yourselves a favor by going to this website (there are many others)... http://www.engineeringtoolbox.com/radiation-heat-transfer-d_431.html q = ε σ (Th4 - Tc4) Ac ...and note that radiation loss depends on two distinct temperatures. That of the emiter AND the receiver. That is your last heads up from me. See ya. -
thepoodlebites at 06:10 AM on 29 December 2010It's Pacific Decadal Oscillation
#39 Why does NASA's Global Land-Ocean Temperature Index differ so much from satellite Weekly Global SST anomaly plots and UAH Global Average Tropospheric Temperature plots? Specifically, compare 2008 data points. The NASA plot shows (+.42) but both weekly SST anomaly and lower tropospheric measurements were near or below zero in 2008. We could be headed back down to 2008 levels or lower, considering the switch from El Nino to La Nina, and current -PDO, -NAO, although the SST website says La Nina seems to be bottoming out. The 2010 El Nino was similar to 1998 and contributed to the higher global average in the first half of 2010, just like in 1998. I tried to provide web links but had trouble getting them to show up properly in the preview. The SST data are from bobtisdale.blogspot.com, Roy Spencer provides the lower tropospheric data, UAH. -
Paul D at 06:04 AM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
HR: "Do crops get damaged by weather or climate?" Erm, I live in the UK and often listen to a BBC radio programme called 'farming today'. Firstly lets start with the weather on the South Coast from Sept through to Dec 2010. Basically from Sept to Nov, it was incredibly warm for the time of year. Secondly the warm weather generally has resulted in bumper crops in some cases, the main problem is harvesting vegetables (eg. brussel sprouts). According to a organic farmer on 'farming today', he can harvest most of his crop at temperatures down to -10. Below that, the crop rots when it thaws. Actually what we do have in the UK is crop migration due to warming, which is why Camel Valley vineyard in Cornwall no longer has to use poly-tunnels to protect their vines. You also seem to have forgotten that Wheat prices have shot through the roof due to Russian droughts and wild fires. As has been stated in previous comments, a graph of crop yields has little to do with eventual production figures, carry over etc. The subject of climate change impact on farming and species is complicated. Simple statistics do not show crop migration (farmers adapting to changing climate) and other issues. UK organisations such as the Tree Council and the Forestry Commission are looking at trees that will grow in changing climates in the UK, including Oaks that have adapted to warmer climates in France.Moderator Response: Additional discussion of crops must move to a more relevant thread such as "It's Not Bad." -
dana1981 at 06:01 AM on 29 December 2010A Positive Outlook For Clouds
Nick - water vapor is a significantly less powerful greenhouse gas than CO2. It's just more prevalent in the Earth's atmosphere, thus it accounts for more of the greenhouse effect. Atmospheric water vapor will indeed increase as the planet warms - there's really no question about that. 'Skeptics' like Lindzen simply postulate that the negative cloud feedback will be so strong as to overwhelm both the CO2 forcing and all positive feedbacks, including water vapor. I can't speak for how sure Lindzen is, but I think the evidence clearly shows that we would be unwise to put our eggs in the 'cloud feedbacks will save us' basket. -
Nick Palmer at 05:26 AM on 29 December 2010A Positive Outlook For Clouds
If we speculate that there will be more clouds around as a feedback to CO2 induced warming, there will also be more water vapour around (needed to form them). Leaving aside the reflectivity due to the clouds, there will be plenty more water vapour (uncondensed) in the areas between and under the clouds. I am probably showing the limits of my knowledge here, but, as water vapour is a more powerful greenhouse gas than CO2, have Lindzen et al taken into account that whilst his hypothesized "Iris" like effect is reducing the radiative forcing, the increased water vapour necessary to generate the extra "Iris" clouds will be increasing the forcing right back up again? How sure is Prof. Lindzen which effect will dominate? Anyone? -
Phila at 05:19 AM on 29 December 2010Did Global Warming stop in
1998,1995,2002,2007, 2010?
Albatross: Now either you support that trickery or you do not. So, where do you stand on that? I think HR's use of a cherrypicked FAO graph answers that question for him. Meanwhile, FAO's 2009 Profile for Climate Change notes that "Climate change negatively affects the basic elements of food production, such as soil, water and biodiversity." Furthermore, "it affects all four dimensions of food security: food availability, food accessibility, the stability of the food supply and the ability of consumers to utilize food including food safety and nutritional value." Therefore, "Action is needed now, [because] inaction will significantly increase future costs." In other words, FAO seems to agree with this article's claim that AGW is "more than enough to disrupt weather systems and cause severe damage to crops and human populations."
Prev 2018 2019 2020 2021 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 Next































 Arguments
Arguments



























