Recent Comments
Prev 702 703 704 705 706 707 708 709 710 711 712 713 714 715 716 717 Next
Comments 35451 to 35500:
-
saileshrao at 03:49 AM on 10 September 2014When their research has social implications, how should climate scientists get involved?
Tom Curtis @29:
I was also under the impression that eggs and milk are low-impact foods and was lacto-ovo-vegetarian until 6 years ago before I realized that those are "low-impact" foods only because of how we did the accounting. The chickens that lay the eggs and the male chicks that are ground up as by-products of the egg industry are all being accounted for by the chicken nugget consumers and the cows that produce the milk are all being accounted for by the hamburger eaters. India has a proliferation of cattle (320 million heads of cattle vs. 90 million in the US) which are literally eating up the forests, because a lot of Indians drink milk, but not many consume beef and averse to the killing of cattle.
The egg and dairy consumers depend upon chicken and beef consumers to clean up after them or else their foods have higher impact than chicken and beef consumption itself. -
Tom Curtis at 03:08 AM on 10 September 2014When their research has social implications, how should climate scientists get involved?
saileshrao @26:
"...an entirely locally sourced, plant-based, Vegan Fall meeting..."
(My emphasis)
Apparently not even eggs, a low carbon, low ecological impact food if ever there was one are acceptable. That underlines the point that saileshao is pursuing this agenda for ideological rather than scientific or ethical reasons.
-
saileshrao at 02:54 AM on 10 September 2014When their research has social implications, how should climate scientists get involved?
Rob Honeycutt @27:
Will do, I'll send the AGU Fall Meeting planning committee another reminder today. The caterers actually gave me the impression that I was the first one from the AGU to contact them and wanted to know details on what we're looking for, breakfasts, breaks, lunch, dinner, etc.
Thank you all for your encouragement, support and stimulating discussions on Skeptical Science. We'll get through this together...
-
Rob Honeycutt at 01:32 AM on 10 September 2014When their research has social implications, how should climate scientists get involved?
Give them a chance, saileshrao. Your issue is likely number 228 of 1000 other things they have to get done in preparation for the December meetings. And I wouldn't be surprised if the decision on catering was made months ago, and this year can't be changed one way or another.
You need to have patience and tenacity for the things you believe are important.
-
saileshrao at 01:14 AM on 10 September 2014When their research has social implications, how should climate scientists get involved?
Rob Honeycutt @25:
The Moscone Center caterer, SAVOR Catering, has already assured me that if the AGU Fall Meeting planning committee wishes to organize an entirely locally sourced, plant-based, Vegan Fall meeting, they are ready and able to do that.
The decision is squarely with the AGU planning committee now. I have already written to every member of the AGU Fall Meeting planning committee and have received no replies whatsoever from any of them. I have reluctantly concluded that there seems to be some kind of blockage in our mental processes when it comes to connecting our personal habits with the environmental catastrophes that we study. -
2014 SkS Weekly Digest #36
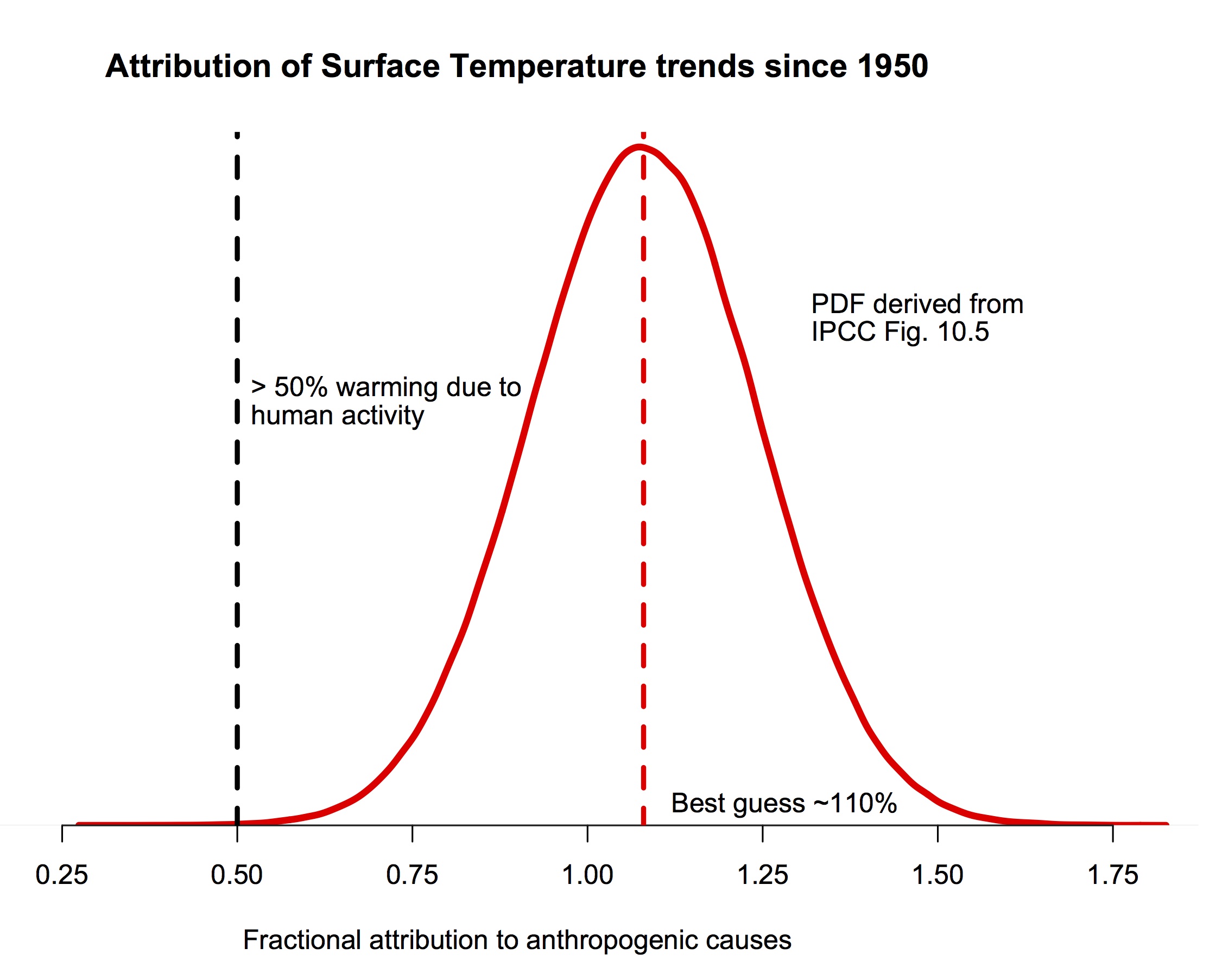
jim - Yes,Tung and Zhou is part of the literature considered in the IPCC reports. But note that it is only one piece of the literature, that there are other works using similar and quite different methods that find the anthropogenic contribution to be much higher. In fact, there are papers referenced in AR5 showing much lower and much higher percentages.
But in looking at all of the literature, and the uncertainties expressed in those works, the IPCC has reported a PDF as shown above. It would be completely inappropriate to ignore the body of evidence in favor of a single paper.
[ Side note: IMO most of the estimates of AMO attribution are flawed. In particular, a linear detrending of North Atlantic temps to identify AMO is wholly inappropriate in the face of other factors/forcings being non-linear over the periods of interest, linear detrending doesn't correctly remove forcings. If you are looking for variability you must first accurately subtract knowns and only then look at unknown contributions, or you will misattribute the remaining forcings to the unknowns. T&Z 2013 fails on this point. ]
-
Lionel A at 00:44 AM on 10 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Congratulations team on another world class utility that shows up the opposition.
-
Lionel A at 00:43 AM on 10 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Yes Lars, I spotted him a few hours back, before noon UK time, looking grim with arms folded. Will we see Lindzen, Spencer or Michaels soon, oh and Soon too?
BTW I first started looking at the graphic with Firefox on a Linux box, Ubuntu, and have more difficulty getting the name up and arm wave. It does work but not quite as slick as on a Win box, with Firefox.
I was wondering about those rotate buttons too, they didn't show up on Linux, the monitor here is at the limits for brightness and contrast, but now I what I am looking for I find them if hovering over the patch where they are, having just located them on the Win box.
-
Tom Curtis at 00:39 AM on 10 September 20142014 SkS Weekly Digest #36
herrhund @6,
"DSL, well isn't that what sciense is about? Ongoing discussion has to be allowed and is a demand of science."
Sorry, you only get to play that card if the paper is published in peer review, or is the official report of an internationally recognized scientific body.
There are too many kooks out there to expect academics to respond to every pet peeve produced by political hacks, politically motivated "think tanks", and other web crazies. If they think they have something of substance, let them publish. If they have not published, and you think they have something of substance, you specify what it is and why it is substantial.
-
2014 SkS Weekly Digest #36
herrhund: "I am sure they have no problem to responding to some of the points in the paper from the global warming policy foundation."
They've responded. You haven't looked. And, no, science isn't required to answer every publicly displayed argument against it. If that were the case, no science would ever get accomplished. GWPF is designed to shape public opinion, not be scientifically accurate. Its writers can write just about anything they want without facing scrutiny. The general public certainly won't scrutinize, and scientists, for the most part, completely understand what the GWPF is all about and treat it accordingly (by ignoring it).
Moderator Response:[PS] It would be better if any further discussion on this was moved to the thread pointed to by DSL. Herrhund - in the interests of a reasonable discussion, it would be best if you picked the point in the GWPF "paper" that you found most compelling (a criticism of the method used that would alter the conclusions of the paper) and that is not already answered.
-
jim10940 at 00:15 AM on 10 September 20142014 SkS Weekly Digest #36
Thank you scaddenp and KR for the references. In reading Chapter 10 of the IPCC report I see that Tung and Zhou (2013) is referenced quite a bit particularly in section 10.3.1.1.1. Here is a quote from the end of that section:
Some studies have suggested that the warming is a response to the AMO (Schlesinger and Ramankutty, 1994; Polyakov et al., 2005; Knight et al., 2006; Tung and Zhou, 2013), or a large but random expression of internal variability (Bengtsson et al., 2006; Wood and Overland, 2010). Knight et al. (2009)
diagnose a shift from the negative to the positive phase of the AMO from 1910 to 1940, a mode of circulation that is estimated to contribute approximately 0.1°C, trough to peak, to GMST (Knight et al., 2005). Nonetheless, these studies do not challenge the AR4 assessment that external forcing very likely made a contribution to the warming over this period. In conclusion, the early 20th century warming is very unlikely to
be due to internal variability alone. It remains difficult to quantify the contribution to this warming from internal variability, natural forcing and anthropogenic forcing, due to forcing and response uncertainties and incomplete observational coverage.Now my reading of the chapter would be that there is anthropogenic warming but the contribution from internal variability and anthropogenic forcing is uncertain. I looked at the Tung and Zhou 2013 paper and they claim that "Quantitatively, the recurrent multidecadal internal variability, often underestimated in attribution studies, accounts for 40% of the observed recent 50-y warming trend."
My question is how to treat that body of literature in the above quote? Its very odd that this literature is included here and supports significant contribution of internal variability to the 20th century warming and yet the chart fig. 10.5 shows mostly a very small contribution from natural and internal variability. So obviously it has been discounted in the IPCC summary. But it is also not explicitly stated that these authors are are wrong in their conclusions. I am uneasy about this. The authors statements should be refuted completely or it is a reasonable opinion to hold that anywhere from 0 to 50 percent of the observed 20th century warming is from internal variability.
I also want to acknowledge that is a very good point that the assumption that the variability is all internal is an open question.
My own position right now is that there is 0 contribution of natural variability to the overall 20th century trend. And yet these above mentioned published authors hold a different opinion. I have to say claiming up to 50% contribution from internal variability is a reasonable position.
-
herrhund at 00:14 AM on 10 September 20142014 SkS Weekly Digest #36
DSL, well isn't that what sciense is about? Ongoing discussion has to be allowed and is a demand of science. There is always a chance that a study is not correct - to act like something is 100% sure is not allowed in scientific methodes, acting like it disqualifies whoever claims to know something for 100%.
If the method of the consensus project is well done according to scientific standards - I am sure they have no problem to responding to some of the points in the paper from the global warming policy foundation.Moderator Response:[JH] You are already skating on the thin ice of sloganeering which is prohibited by the SkS Comments Policy.
Please note that posting comments here at SkS is a privilege, not a right. This privilege can be rescinded if the posting individual treats adherence to the Comments Policy as optional, rather than the mandatory condition of participating in this online forum.
Please take the time to review the policy and ensure future comments are in full compliance with it. Thanks for your understanding and compliance in this matter.
-
Falk at 23:19 PM on 9 September 2014Rising Ocean Temperature: Is the Pacific Ocean Calling the Shots?
In the label of Fig. 1 you write that it shows the Ocean heating rates. Are the rates or the total heat content plottet in the graphs? I took it for the absolute change of heat content relative to a time average.
I find it interesting to see that the HOC is increasing the strongest around antartica in the deep while the see ice area (not volume) shows slight increase and surface temperature is more or less constant. Are there any effects on ocean currents expected from a changing gradient in this region?
-
DSL at 21:55 PM on 9 September 20142014 SkS Weekly Digest #36
herrhund, do you think it's worth responding to? Do you find its method to be sound?
-
Lars Karlsson at 20:01 PM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
I think I can spot Christy now (without any quote).
-
Kevin C at 17:16 PM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
I wanted the Mojib Latif quote to be this one:
'If my name were not Mojib Latif, my name would be global warming'
If I remember correctly in response to some contrarian claiming that his work supported them.
-
GSR at 17:13 PM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Rob Honeycutt @22. A graph is just a Rorschach test to Anthony.
-
herrhund at 17:03 PM on 9 September 20142014 SkS Weekly Digest #36
Hey guys,
are you going to comment on this paper from the global warming policy foundation?
http://www.thegwpf.org/content/uploads/2014/09/Warming-consensus-and-it-critics.pdf
Quote:
"While Cook’s approach appears to owe more to public relations or propaganda than the scientific method, there is little doubt that there is a scientific consensus, albeit not the one that the authors of the paper have led people to believe exists. The consensus as described by Cook et al. is virtually meaningless and tells us nothing about the current state of scientific opinion beyond the trivial observation that carbon dioxideis a greenhouse gas and that human activities have warmed the planet to some unspecified extent. The figure of 97% is entirely discredited, whatever the nature of the consensus."
-
BillTheCat at 15:25 PM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
http://www.sciencemag.org/content/345/6199/897
Varying planetary heat sink led to global-warming slowdown and acceleration
Global warming seems to have paused over the past 15 years while the deep ocean takes the heat instead. The thermal capacity of the oceans far exceeds that of the atmosphere, so the oceans can store up to 90% of the heat buildup caused by increased concentrations of greenhouse gases such as carbon dioxide. Chen and Tung used observational data to trace the pathways of recent ocean heating. They conclude that the deep Atlantic and Southern Oceans, but not the Pacific, have absorbed the excess heat that would otherwise have fueled continued warming.
-
wili at 12:58 PM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
So how many of these folks do people recognize (at least by name, if not by caricature)? I seem to recognize the names of about a third of them, so far (though it is difficult to pull up some of the figures who are partly behind others).
Response:You can use the rotate buttons in the top corners to move the characters around, if someone is obscured.
-
wili at 12:54 PM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
I wonder if SkS might consider publishing 'Climate Scientist Cards' (like baseball cards, except, well, you know). Some of our local coops have done this for local small farmers. It kind of gets across the idea how skewed our priorities are in our culture, and who are true heroes are.
The cards could include these caricatures, the quotes, and a short blurb--maybe stats on publications...
-
David Kirtley at 11:32 AM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Update to my comment at #14 above.
The 97 Hours website has been updated so that when you click on one of the lit-up cartoons the quote bubble will pop-up and stay up - so you can click on the scientist's bio page. There is also now a link in the lower right which goes to the source of the scientist's quote.
Have fun!
-
Tom Curtis at 09:08 AM on 9 September 2014Sea level rise is exaggerated
TD inline @202

Cristobal JASL trend, 1907-2010: 1.5861 mm/yr. Note that there is a large dip in sea level around 2000, with a rapid recovery (nearby stations show trends of 13.788 and 13.707 mm per year in the period of recovery) such that by carefull enough chery picking you might find a period with near zero trend depite the rapid overall rise.

Balboa JASL 1907-2010: 1.5494 mm/year (previous link). In both cases extending the data beyond that held for the PSMSL stations increases the rate of sea level rise. That strongly suggests that any lack of sea level rise Maui claims is, at best, the result of cherry picking.
-
Glenn Tamblyn at 07:52 AM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Donny
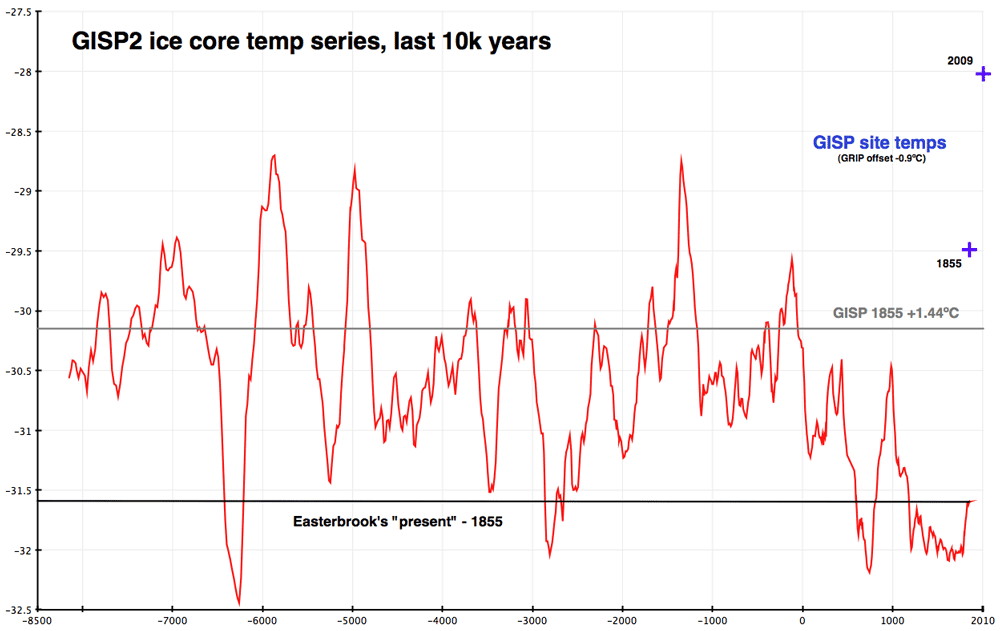
I assume you are reerring to the big change at around 6500 BP. This period, the major cooling ad warming is called the 8.2 kiloyear event. The main theory for its cause is that it was the final collapse of Lake Agassiz. Agssiz and its sister Lake Ojibway were massive glacial lakes in the northern US and central Canada that formed as the Laurentide ice sheet melted. Probably held more fresh water than all lakes on earth today.
The main theory is that repeatedly as the ice sheets melted these lakes were prone to ice wall collapse floods, dumping huge amounts fo fresh water. When these bursts of fresh water flooded out into the Arctic or North Atlantic the change in salinity they caused triggered a slowdown/collapse in the Atlantic Meridinal Overturning Circulation- the current system that includes the Gulf Stream that makes wester Europe artificially warm. If the current collapses then the north atlantic basin can cool signiicantly and then warms again when the current restablishes.
The 8.2 Kyr event is thought to have resulted from the last collapse of Lake Agassiz - there were earlier floods triggering previous events like this that come from earlier in the core, not shown on this graph.
Importantly, these sort of events appear very differently in the Antarcic cores and other studies with the same period being perhaps warmer. Which is what we might expect - if heat isn't being moved northward then there should be more heat remaining in the south.
The cores aren't recording global events so much as local and regional ones. Standard denier tosh to suggest, imply, or 'leave the reader to form their own view' that black is white through cherry picking the data they use.
-
2014 SkS Weekly Digest #36
jim - Depends on the period you are looking at, on whether you are examining sufficient data for statistical significance, etc. The period 1985-1999, for example, is 15 years (too short for significance, as are the last 15-17 years), showing a warming rate of 0.23 C/decade, while the statistically significant period 1984-2014 shows a warming rate of 0.167 C/decade. Short term variations definitely added to warming in the 1990's (albeit without statistical significance).
Longer term, the AMO for example appears to have had a cooling influence in recent decades (Mann et al 2014). There seems to be no way to support natural variation as a majority cause in recent warming.
"...does that mean that the warming attributed to anthropogenic carbon is overestimated?" - No, it means that there is natural variability plus the anthropogenic forcing trend, hardly a surprising result. See IPCC AR5 WG1, Chapter 10, Detection and Attribution of Climate Change, for the current state of the science.
As noted on RealClimate wrt Chen, Tung, and others, "Nobody has any problems with the idea that multi-decadal internal variability might be important. The problem with many studies on this topic is the assumption that all multi-decadal variability is internal. This is very much an open question."
-
scaddenp at 07:00 AM on 9 September 20142014 SkS Weekly Digest #36
Jim, try a read of Double Standard. Note also that the internal variability strongly affects surface temperature (ENSO in particular), but it doesnt do much to OHC. I would say that pretty much all variations in OHC are due to change in forcings.
-
jim10940 at 05:48 AM on 9 September 20142014 SkS Weekly Digest #36
If the hiatus is due to internal variation of the climate system does that imply that previous warming must be partially attributed to internal variation? And further does that mean that the warming attributed to anthropogenic carbon is overestimated?
I refer to Matt Ridley's argument and his defense here:
http://www.mattridley.co.uk/blog/whatever-happened-to-global-warming.aspxI found convincing the email he quoted from the Chen and Tung (2014) authors supporting his interpretation of their paper.
-
Composer99 at 04:37 AM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
I must say, adding the guitar into Dr Alley's cartoon character is a nice touch.
-
Marco at 03:10 AM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
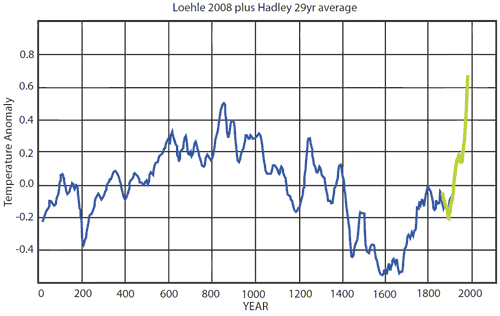
Rob, as also pointed out at Hotwhopper, Watts actually put up Loehle 2007, before all the corrections were made.
-
Rob Honeycutt at 02:38 AM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Marco @18... Also, Watts tries to pull out Loehle's work to make up for his posting such a thoroughly debunked graph of GISP2. But in my first SkS article I took that one on and created the graph below with Dr Loehle's assistance.
-
Rob Honeycutt at 02:02 AM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Donny... You have to bear in mind that that graph represents only one small part of the globe. It is a regional record of temperature at the Greenland summit. Also bear in mind where the Greenland summit is. It's well above the arctic circle and thus it's where we expect to see much more rapid swings in temperature.
I'm really amazed that Watts has posted that version of the GISP2 data. He totally knows that the it's wrong where it states that the year 2000 = "present." That should give you a sense of how much Watts cares about accuracy and truth. In fact, of all the versions of the GISP2 data floating around the denialsphere, that one is probably the most misleading of all.
-
Maui at 01:59 AM on 9 September 2014Sea level rise is exaggerated
It is curious this controversy on a scientific level. "They have a millimeter measurement" for 68 years ... every day ... well documented ... Panama Canal.
Repeat ... for 68 years
Without any pressure from powerful groups and only practical for the normal functioning of locks filing purposes.
The levels of the Pacific and the Atlantic, have been unchanged in the last 68 years.Give a bit of a laugh to see how each to defend a theory and make specific studies in specific geographic areas and draw overall conclusions.
.
Governments need to collect more taxes, and for that... we have to blame something.Moderator Response:[TD] Please provide a citation for your claim. NOAA's Sea Level Trends page shows trend of increase since 1908/1909 on both ends of the canal. (Click the arrows on that page to see more info, and in the resulting dialog window click the "Linear Trend" link.)
-
Donny at 01:54 AM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
KR looking at the graph above it looks like there was rapid warming 6300 years ago of more than 3 degrees in about a 200 year span.... do they have theories as to what caused that rapid warming?
-
97 hours of consensus: caricatures and quotes from 97 scientists
Donny - That's one of the most misrepresented data sets on the climate blogosphere. See the Crux of a Core series here on SkS.
Long story short: it's a local record of a particular location in Greenland, not a global proxy, often mis-graphed with incorrect proxy endpoints, and shown with a noteworthy lack of the most recent temperatures:
Good data, deceptive presentations, ridiculous interpretations.
-
Marco at 01:18 AM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Donny, see http://skepticalscience.com/10000-years-warmer.htm
No surprise to see WUWT not caring (see their "update") about using, once again, a falsified graph.
-
Rob Honeycutt at 01:08 AM on 9 September 2014When their research has social implications, how should climate scientists get involved?
saileshrao... If you're an AGU member and are genuinely interested in this issue, then I'd suggest you approach the Moscone Center people about it. AGU is not a caterer, nor does AGU even select a caterer, for the events. Whoever does event organizing at AGU is, likely, merely selecting which services the Moscone Center offers. It's unlikely AGU has an option to choose an outside catering service for events. That would be the exclusive service of Moscone Center.
That said, this is San Francisco. I would imagine the management at Moscone Center would be open to hearing your suggestions about offering low carbon meal packages for events. Only at that point would AGU even have the option to do what you're suggesting they do.
If you think it's an important issue, it's certainly within your power to help make it happen.
-
Donny at 00:30 AM on 9 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Can someone please tell me if this Greenland ice core graph is real or crap? Not sure who to believe.
wattsupwiththat.com/2014/09/08/monday-mirthiness-97-hours-97-opinions-97-consensus/
-
Dikran Marsupial at 18:07 PM on 8 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Jenna, I suspect there are a handful professors who don't have a PhD (for instance because they have built up an impressive track record of industrial research before moving into academia) but I would suspect the number would be exceedingly small, especially as it is possible to get a "PhD by publication".
-
Ashton at 14:56 PM on 8 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Jenna Professor is an academic title used by universities. Most professors have a track record of attracting grants and publishing in the peer reviewed literature and are considered experts in their field. Drs in this context have a PhD but may not work in a university so are not referred to as Professor even though their scientific output is similar to those of "Professors". And of course there are may scientists working in university who also have excellent credentials but are not professors as there are only a limited number of professorships available
-
saileshrao at 14:08 PM on 8 September 2014When their research has social implications, how should climate scientists get involved?
Tom Curtis @23:
The carbon cycle is not the only biogeophysical cycle that humans have altered among the planetary life support systems. As a member of AGU, I'm concerned about all the cycles that we have messed up, not just the carbon cycle.
As Prof. Will Steffen said, "Climate change is one of many global changes that are destabilising our planetary life support system. It is ultimately a question of core values. Can we change our core values rapidly enough – and decisively enough – to halt our slide towards collapse? That is humanity’s most important question in the 21st century!"
As scientists, we are very good at rationalizing any set of core values, but then we should expect the general public to get very good at ignoring our pet peeves. -
David Kirtley at 11:28 AM on 8 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Each scientist's cartoon and quote page (follow BaerbelW's link or instructions above at comment #7) here on SkS has a link to that scientist's bio page. Usually hosted by their university, etc.
Each hour's featured scientist on the 97Hours website (http://skepticalscience.com/nsh/?#) has the same bio link.
Also, when you hover over the smaller figures which are already "lit up" on the "turntable", and click your mouse that scientist's quote balloon will pop up. The bio link is also in that bubble but there is a trick to get to it. Click and hold your mouse button and drag over to the underlined link, release the button and a new tab/window will open with the bio page.Have fun!
-
SteveS at 11:19 AM on 8 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Jenna, I have found the rotation and pan arrows to be helpful sometimes in accessing a particular scientist. I think those buttons give a very cool effect, so congratulations to the people who worked on this.
-
DSL at 11:13 AM on 8 September 201497 hours of consensus: caricatures and quotes from 97 scientists
For example, David Karoly.
-
DSL at 11:11 AM on 8 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Jenna, the professors are scientists who regularly publish in their respective sub-disciplines. It would be cool to get a google scholar link for each of them (or a link to their CVs).
-
jenna at 11:01 AM on 8 September 201497 hours of consensus: caricatures and quotes from 97 scientists
I have 2 observations;
1) it's vewry difficult to get some of the figures to respond to your mouse-over. I'm not sure what the problem is, do we need to rotate the screen?
2) there seem to be too many "professors" and not enough "scientists" (as in dr.'s, etc). Or are the professors really scientists?
Jen.
-
Ken in Oz at 09:51 AM on 8 September 20142014 SkS Weekly News Roundup #36B
jja should that perhaps be "Climate change threatenst to put the fight against hunger back by Millenia"?
-
Tom Curtis at 09:43 AM on 8 September 2014When their research has social implications, how should climate scientists get involved?
saileshrao @19, a reasonable estimate for revving car engines for an hour is 5 liters of petrol consumed (based on a 1.8 liter, 4 cylinder engine), with CO2 emmisions of 11.5 Kg. That in turn is half the CO2e emissions of 1 kg of grass fed beef, which comes in at 19.2 Kg per live kg of beef for the worst category (grass fed) in the Midwestern USA. Even serving 500 g steaks means the beef is comparable to just one hours of "revved engines", and for the more reasonable 350 or 200 g steaks, the advantage is entirely with the beef. Of course, the calculations above do not include CO2 generated in transporting and cooking the beef, but nor does it include that in transporting, refening and than further transporting the petrol.
Of course, there is just one banquet at an AGU, and you propose an equivalent of 15 hours revved engines as the CO2 equivalent of that banquet. Clearly you are not arguing this from science, or anything approaching a factual basis.
Further, the AGU already advertizes its attempts to support sutainability, mostly through its choice of convention center which will by itself reduce emissions generated from the meeting by a greater amount than eliminating beef from the menu. They further recommend that attendee's use the BART transport system, which will save CO2 emmissions more than that of a steak meal for each round trip. These steps are visible, more effective, readilly associated with a concern regarding CO2 emissions and do not advertize a hair shirt mentality in the same way that banning beef from the banquet would do. That later point, however, makes them insufficient from saileshao's point of view.
saileshao @21, "foregoing the constant economic growth paradigm" is not necessary to tackle climate change and should not be coupled to reducing CO2 emissions as a strategy. Doing so merely encumbers the later making it far less politically achievable. Once again, hooking your particular political agenda to concern about AGW acts only to the detriment of the later and is not the strategy that shoud be pursued by those whose primary concern is ensuring AGW is controlled.
-
Trakar at 06:53 AM on 8 September 2014When their research has social implications, how should climate scientists get involved?
Personally, most of those I know, regardless of their political persuasion, understand and accept the basic mainstream science. While this is interesting, it leaves a lot of room for social/public policy choices and decisions. I'd rather start hearing more about the range of options and trade-offs with respect to individual choices and decisions, neighborhood/local planning, as well as State and Federal options.
If enough people are responding at individual and local levels, we can drag the higher levels of social architecture along for the ride. Too many are interested in working on national and state level politics, without much attention to their personal and local level decisions and options. Too many view this as a black or white issue that one political "side" has the monopoly on. As in most such issues the reality is closer to being that there is one side that is generally much less wrong about what the science says. In fact, there are many traditionally fiscally conservative public policy positions, such as revenue neutral carbon taxes, that ahould, and probably will, play vitally important roles in addressing AGW climate change adaptation and mitigation.
We will never get past the "should we do something" stage and fully into the "what should we do" stage, if we don't start defining the options that everyone has at each level of decision making.
-
wili at 06:49 AM on 8 September 201497 hours of consensus: caricatures and quotes from 97 scientists
Is this great initiative being intentionally coordinated with the "Disruption" screenings being held tonight around the country?
"Disruption" film: grassroots global revolt a key answer to CC
-
saileshrao at 06:08 AM on 8 September 2014When their research has social implications, how should climate scientists get involved?
Rob Honeycutt @20
Re: "Simple taxation works wonders to alter broad consumer behavior."
I don't see governments all over the world and the corporations who control them in our capitalist system foregoing the constant economic growth paradigm anytime soon. Therefore taxation policies that alter peoples' behavior so that they become net contributors to ecosystems instead of net consumers is a distant dream. This is not as easy a problem as getting people to switch to reusable shopping bags, which is why governments have been punting on this issue for the past two decades.
We're facing the same intractable problem worldwide that Gandhi faced in India in 1915: an entrenched power structure that is utterly impervious to reason. Gandhi tackled it with voluntary grassroots actions.
Besides, why wait to do the right thing at AGU meetings just because we haven't yet received price signals not to do the wrong thing? The IPCC AR5, Chapter 11, is unequivocal that the consumption of animal foods at present levels is unsustainable. Please take a look at Fig. 11.9 to see the land use component and the energy flow of animal food products vs. the land still remaining as pristine forest and the energy flow of plant food products. That figure illustrates why Nature has a loaded gun pointed at our heads, saying, "Change your conduct or it's your life!". And those stats are from 2000 and it has only gotten worse since then!
Prev 702 703 704 705 706 707 708 709 710 711 712 713 714 715 716 717 Next































 Arguments
Arguments