How do we know more CO2 is causing warming?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
An enhanced greenhouse effect from CO2 has been confirmed by multiple lines of empirical evidence. |
|||||||
Climate Myth...
Increasing CO2 has little to no effect
"While major green house gas H2O substantially warms the Earth, minor green house gases such as CO2 have little effect.... The 6-fold increase in hydrocarbon use since 1940 has had no noticeable effect on atmospheric temperature ... " (Environmental Effects of Increased Atmospheric Carbon Dioxide)
At-a-glance
To make a statement like, "minor greenhouse gases such as CO2 have little effect", is to ignore 160 years of science history. So let's look at who figured out the heat-trapping properties of carbon dioxide and when.
Experiments involving various gas mixtures had demonstrated the heat-trapping properties of water vapour, CO2 and methane in the 1850s. But those effects were yet to be quantified - there were no meaningful numbers. It was to be another 40 years before that happened.
Swedish scientist Svante Arrhenius (1859-1927) was the person who crunched the numbers. The results were presented in a remarkable paper, "On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground", in 1896.
The many calculations in the 1896 paper include estimates of the amounts of CO2 increase or decrease required to drive the climate into a different state. One example used was the Hothouse climate of the Cenozoic, around 50 million years ago. Another was the glaciations of the last few hundred millennia.
To get a temperature rise of 8-9°C in the Arctic, Arrhenius calculated that CO2 levels would have to increase by 2.5 to 3 times 1890s levels. To lower the temperature 4–5°C to return to glacial conditions, he calculated a drop in CO2 was needed of 0.62-0.55 times 1890s levels.
We know CO2 levels in the 1890s from ice-core data. They were around 295 ppm. Let's do the sums. A reduction factor of 0.55 to 0.62 on 295 ppm gives 162.2-183.9 ppm. Modern ice-core measurements representing the past 800,000 years show that in glacial periods, CO2 levels fell to 170-180 ppm.
What we now know due to additional research since 1896 when Arrhenius worked on this, is that CO2 was an essential 'amplifying feedback'. That means changes triggered by long term, cyclic variations in Earth's orbit cause warming or cooling and CO2 release or entrapment in turn. Those changes in CO2 levels affected the strength of Earth's greenhouse effect. Changes in the strength of the greenhouse effect then completed the job of pushing conditions from interglacial to glacial - or vice-versa.
Arrhenius also made an important point regarding water vapour: "From observations made during balloon voyages, we know also that the distribution of the aqueous vapour may be very irregular, and different from the ideal mean distribution." This statement holds true today: water vapour is a greenhouse gas but because water exists in gas, liquid and solid forms in the atmosphere, it is continually cycling in and out of the air. It is distributed in a highly uneven fashion and is uncommon in the upper atmosphere. That's where it differs from CO2.
Once CO2 is up there, it's up there for a long time. As a consequence it has a pretty even distribution: 'well-mixed' is the term. As Arrhenius quantified all that time ago, once it's up there it constantly absorbs and re-radiates heat in all directions. That's why dumping 44 billion tons of it into our atmosphere in just one year (2019 - IPCC Sixth Assessment Report 2022) is a really bad idea.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
Good scientific theories are said to have ‘predictive power’. In other words, armed only with a theory, we should be able to make predictions about a subject. If the theory’s any good, the predictions will come true.
Here’s an example: when the Periodic Table of the chemical elements was proposed in 1869, many elements were yet to be discovered. Using the theory behind the Periodic Table, the Russian chemist Dmitri Mendeleev was able to predict the properties of germanium, gallium and scandium prior to their discovery in 1886, 1875 and 1879 respectively. His predictions were found to be correct.
The effect on Earth's greenhouse effect of adding man-made CO2 is predicted in the theory of greenhouse gases. This theory was first proposed by Swedish scientist Svante Arrhenius in 1896, based on earlier work by Fourier, Foote and Tyndall. Many scientists have refined the theory since Arrhenius published his work in 1896. Nearly all have reached the same conclusion: if we increase the amount of greenhouse gases in the atmosphere, the Earth will warm up.
Where there is less agreement is with respect to the exact amount of warming. This issue is called 'climate sensitivity', the amount the temperatures will increase if CO2 is doubled from pre-industrial levels. Climate models have predicted the least temperature rise would be on average 1.65°C (2.97°F) , but upper estimates vary a lot, averaging 5.2°C (9.36°F). Current best estimates are for a rise of around 3°C (5.4°F), with a likely maximum of 4.5°C (8.1°F). A key reason for this range of outcomes is because of the large number of potential climate feedbacks and their variable interactions with one another. Put simply, some are much better understood than others.
What Goes Down…
The greenhouse effect works like this: Energy arrives from the sun in the form of visible light and ultraviolet radiation. The Earth then emits some of this energy as infrared radiation. Greenhouse gases in the atmosphere 'capture' some of this heat, then re-emit it in all directions - including back to the Earth's surface.
Through this process, CO2 and other greenhouse gases keep the Earth’s surface 33°Celsius (59.4°F) warmer than it would be without them. We have added 42% more CO2, and temperatures have gone up. There should be some evidence that links CO2 to the temperature rise.
So far, the average global temperature has gone up by more than 1 degrees C (1.9°F):
"According to an ongoing temperature analysis led by scientists at NASA’s Goddard Institute for Space Studies (GISS), the average global temperature on Earth has increased by at least 1.1° Celsius (1.9° Fahrenheit) since 1880. The majority of the warming has occurred since 1975, at a rate of roughly 0.15 to 0.20°C per decade."
The temperatures are going up, just like the theory predicted. But where’s the connection with CO2, or other greenhouse gases like methane, ozone or nitrous oxide?
The connection can be found in the spectrum of greenhouse radiation. Using high-resolution FTIR spectroscopy, we can measure the exact wavelengths of long-wave (infrared) radiation reaching the ground.
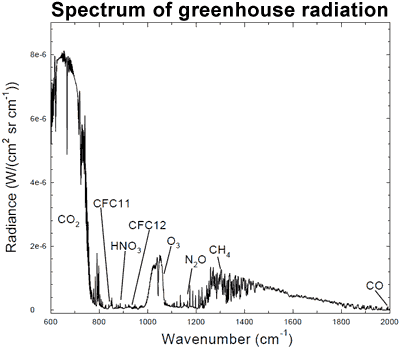
Figure 1: Spectrum of the greenhouse radiation measured at the surface. Greenhouse effect from water vapour is filtered out, showing the contributions of other greenhouse gases (Evans 2006).
Sure enough, we can see that CO2 is adding considerable warming, along with ozone (O3) and methane (CH4). This is called surface radiative forcing, and the measurements are part of the empirical evidence that CO2 is causing the warming.
...Must Go Up
How long has CO2 been contributing to increased warming? According to NASA, “Two-thirds of the warming has occurred since 1975”. Is there a reliable way to identify CO2’s influence on temperatures over that period?
There is: we can measure the wavelengths of long-wave radiation leaving the Earth (upward radiation). Satellites have recorded the Earth's outgoing radiation. We can examine the spectrum of upward long-wave radiation in 1970 and 1997 to see if there are changes.
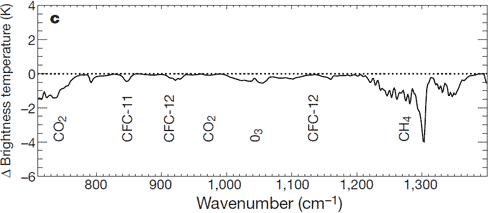
Figure 2: Change in spectrum from 1970 to 1996 due to trace gases. 'Brightness temperature' indicates equivalent blackbody temperature (Harries et al. 2001).
This time, we see that during the period when temperatures increased the most, emissions of upward radiation have decreased through radiative trapping at exactly the same wavenumbers as they increased for downward radiation. The same greenhouse gases are identified: CO2, methane, ozone and so on.
The Empirical Evidence
As temperatures started to rise, scientists became more and more interested in the cause. Many theories were proposed. All save one have fallen by the wayside, discarded for lack of evidence. One theory alone has stood the test of time, strengthened by experiments.
We have known CO2 absorbs and re-emits longwave radiation, since the days of Foote, Tyndall and Arrhenius in the 19th Century. The theory of greenhouse gases predicts that if we increase the proportion of greenhouse gases, more warming will occur.
Scientists have measured the influence of CO2 on both incoming solar energy and outgoing long-wave radiation. Less longwave radiation is escaping to space at the specific wavelengths of greenhouse gases. Increased longwave radiation is measured at the surface of the Earth at the same wavelengths.
Last updated on 16 July 2023 by John Mason. View Archives































 Arguments
Arguments






































In the last paragraph of the previous post it should say "causing less IR radiation to get out"
I dont actually believe you guys are scientists because "actual scientists" such as nasa have proven that climate change is real and that the sun is not too blame and that co2 is the problem
[JH] Your comment seems to be in response to the denier meme statement which the article rebuts. Please read the article.
It makes no sense that satellites should record a lessened flux of radiation to space within the emission band of CO2 when the concentration of CO2 is increasing in the atmosphere. If half of all emissions from CO2 go upward to space, then a higher atmospheric CO2 concentration should result in a greater, not a lesser, flux of radiation to space. The latter should not be at all affected by the storage of IR within Earth's atmospheric system. That only involves the half of radiation that is directed downward. A decrease in flux recorded by satellites is most likely due to an error in recording outward flux. I'd welcome some comments on this. Tx.
davidbennettlaing,
Your argument is an argument from increduility. You need to provide data to support your claim. Since experienced atmospheric chemists and physicists agree that increasing CO2 results in lowered emissions at the CO2 emission bands, it seems more likely that you are incorrect than they are.
The Earth emits black body radiation upward. CO2 absorbs in the same bands that it emits. Energy is re-emitted upward and downward (as you state). The energy emitted upward is reabsorbed at a higher altitude. Energy is re-emitted up and down. Eventually the energy emitted upward escapes to space. This is called the escape altitude. The satalite measures the energy that escapes.
The energy that escapes is emitted from much higher in the atmosphere that the original energy that was emitted from the surface of the Earth. Since as you increase in altitude in the atmosphere it is colder, the energy is emitted from molecules that are colder than the surface molecules. When molecules are colder less energy is emitted. This difference in energy is what the satalite measures. It relates to the difference in temperature between the surface and the atmosphere at the escape altitude.
Increasing the amount of CO2 in the atmosphere causes the escape altitude to increase. That results in lower emissions of energy since it is colder at higher altitudes. The change in temperature with altitude is called the lapse rate. The lapse rate is about 6C per kilometer of altitude. Thus an increase of 100 meters in altitude results in a shift of about 0.6C in emission temperature.
Since this decrease in energy has been measured, it makes no sense for you to object to measured data. An explaination for the change is required.
davidbennetlaing:
You are thinking in terms of "CO2 changes, but nothing affecting emission else does". This is not correct.
Temperature is also a factor. As the temperature profile changes, cooler temperatures tend to cause less IR emission. Also, as the increase in CO2 increasing emissivity, you can get the same emission with a cooler temperature.
At any point in the atmosphere, the upward flux of IR is a combination of what is emitted locally, plus whatever was emitted upwards from lower layers that has not yet been absorbed.
What is seen from space is a rather complex integration of emission from all atmospheric layers, less any absorption by overlying layers. You cannot think of it as a single "emit half up, half down" event. In mathematical terms, it is not a single equation, but a rather large system of equations.
Doing the full math indicates that the lower atmosphere will warm, and the stratosphere will cool, and the view from space will be affected as indicated by the observations michael sweet has indicated.
One of my friends conducted this backyard experiment.
Could anyone explain what the error was with her approach, and whether or not there is a backyard-style experiment that she could do to observe the reradiating properties of CO2.
"So, what I did was take two clear 6L ziplock bags, two cheap thermometers, 2 CO2 cartridges, and a seltzer bottle.
The first bag, I taped the thermometer to the upside of the bag, then filled with normal air using a balloon pump, then measured the temperature (19.4 deg).
The second bag, I taped the thermometer to the upside of the bag, then measured (19.8 deg - remember, they're shit $2 thermometers - note slightly warmer than "control"), then I filled it by releasing two CO2 chargers into it. I then measured the temperature again (19.0 deg) and again a few minutes later (18.7 deg).
I then took them outside and placed them in the sun and measured each over a ten or so minute period. The bag containing CO2 was always colder - between 0.4 to 1.0 degree (it measured 1.4 colder, but I reject that because I was blocking the sun just before I measured).
Each of the two CO2 cannisters has 7.8 g. The density of CO2 at STP is 1.98 kg/m3. For the 2 x 7.8 = 15.6 g, this equates to a volume of 7.88 L. The seltzer bottle holds 1.25 L. So total 7.88 + 2 x 1.25 = 10.38 L Total. The proportion of CO2 in that mix is 7.88/10.38 = approx 76%.
So as you can see, I significantly increased the CO2 content of the air, and the results came back slightly negative re. its effect on temperature."
(Thanks to anyone with the time to respond)
Tristan @331,
I'll hav a bash at an explanation for you.
If we ignore problems of calibration & measurement errors (hearing of the shading of one of the samples is a bit of a worry), the flaws in the as-described experiment are surely quite profound.
If we consider that a bag of gas in a transparent (to visible light) plastic bag will be heated through radiative transfer and thus measuring its temperture will give some indication of that radiative transfer, we are this comparing an Atmosphere bag heated both by sunlight plus terrestrial IR with a CO2 bag containing predominantly CO2. If one bag absorbs more radiation than the other, if the experiment is sensitive enough to show the effect, the more absorption would therefore register a higher temperature.
However, to expect the High-CO2 bag to absorb more radiation is asking a bit much. The sunlight will be warming the oxygen/ozone and any water vapour in the Atmosphere bag. And additionally terrestrial IR will also be warming the atmosphere's CO2, CH4, N2O and again any water vapour in the Atmosphere bag. With the CO2 having replaced pretty-much all these absorbing gases and with all but a small part of sunlight absorbed by CO2, the comparison is asking whether the narrow CO2 IR absorption band when saturated with CO2 will absorb more or less than the atmospheric gases. In full sunlight, I would be surprised if CO2 was that absorbent. This graphic (usually 2 clicks to 'download your attachment') gives some indication of the absorbtion of various atmospheric gases. (I'd doubt whether the scales allow the various areas to be totted up.) But note that, while sunlight-in & IR-out will balance over a 24-hour period, sunlight operates for a shorter time than IR so at midday the sunlight could be four-times more powerful than the IR.
While the experiment isn't ever going to properly reproduce the mechanisms that result from higher CO2 in the atmosphere, as a measure of CO2 absorbtion of IR, the experiment would have a better chance if conducted at night with the thermometers under the bags.
I will try to add to MA Rodger's commentary.
Tristan:
Keep i mind that the only thing that the thermometer tells you is the temperature of the thermometer. Although this may seem a triviality, it is essential to start with this understanding. The next stage in trying to use the thermometer for any practical purpose is to try to get the temperature of the thermometer to match the temperature of the thing you are really interested in. In your experiment, you are interested in the gas inside the bag (comparing air-filled vs. CO2).
A good way to think of the behaviour between the thermometer and its surroundings is to describe the energy balance of the thermometer. What are all the energy flows in and out of the thermometer, and under what conditions will the temperature of the thermometer match the gas in the bag?
The thermometer can have three methods of energy exchange with its surroundings:
So, in your experiment, you want terms 2 and 3 to equal zero to make sure you have the thermometer at the same temperature as the gas in the bag. This only happens if the radiation term is also zero.
If the radiation term is positive, and the evaporation term is zero, then the positive radiation input will make the thermometer warmer than its surroundings. It will heat up until the radiation input is exactly matched by the thermal loss (energy moving from warm thermometer to cooler gas).
Now, how can you get the radiation term to zero when your goal is to see the effect of increased absorption due to CO2? If the CO2-filled bag is absorbing IR radiation in greater quantities than the air-filled bag, then initially it will warm, but after it has warmed the bag/thermometer will also be emitting more IR - which you hope will balance the extra absorbed IR.
There are two catches to this:
Catch #2 is the experiment-killer. You said you performed this out "in the sun". You haven't mentioned a time of day or location, but direct beam solar radiation usually approaches 1000 W/m2 on a nice clear day, and very slight differences in absorbed solar radiation will overwhelm the IR effect you want to see (maybe 1 W/m2?). My guess would be slight differences in the angle of the thermometers, or reflectivity of the system. Perhaps the plastic bag surface reflects a bit of sunlight at certain angles, so slight differences in shape or orientation alter the amount of solar radiation hitting the thermometer.
Controlling for solar radiation error is a critical factor for weather observations of air temperature. Thermometers are usually housed in a Stevenson Screen or other radiation shield. They are also typically well-ventilated (strong air circulation).
You can't "well-ventialte" the air and CO2 in your bags, because that defeats the purpose of getting the elevated CO2 to absorb IR. That leaves a very large factor of solar radiation error, which makes it difficult in your experimental setup to know if you are looking at an IR effect. (You are most likely not.)
Thanks a ton MA And Bob. I appreciate it. I don't have the physics comprehension to address queries regarding that aspect of climate change.
Hopefully my friend pops in herself if she has any follow-ups to your repsonses.
Tristan, adding some points.
When the CO2 is released from the spritzer, the gas will come out somewhat cooled, so that may explain the lower CO2 temperature in the bag. How good an insulator is the plastic, that may impact how easily any temperature difference due to this corrects?
Better would be to set up the entire setup outside in the same environment, and take measurements before you add any gas. Then look at how the temperatures change immediately after you add the gases. Then monitor how they change subsequently after that.
Next, although CO2 absorbs infrared, most of that absorption in the atmosphere takes 100's to 1000's of meters to be 100%. How much will be absorbed over inches?
Next, what is the transmissivity of the plastic - how much radiation passes through the bag? The bags may be transparent to visible light (very high transmissivity) but most materials behave very differently for infrared light. Most naturally occuring materials have extremely low transmissivity to infrared. You would need to research the properties of the plastic involved. Otherwise you are effectively carrying out the experiment with an opaque (to infrared) bag.
The greenhouse effect depends on the behaviour of the entiure atmosphere over vertical distances of many kilometers. Extra absorption by CO2 at the surface is only a small part of any change in the GH effect. The big changes involve how much emission by CO2 changes at high altitude - 10 km or so. It is very hard to model the GH effect with small, surface based setups.
Hi MA and Bob,
Thanks for your responses re. my "plastic bag" study.
I have a few follow-up questions:
MA:
I agree with the general point that there is no calibration. It is backyard science - I did it to try to understand what the effect is at all, not to measure it accurately.
> However, to expect the High-CO2 bag to absorb more radiation is asking a bit much. ... while sunlight-in & IR-out will balance over a 24-hour period, sunlight operates for a shorter time than IR so at midday the sunlight could be four-times more powerful than the IR.
Let me clarify that what you're saying here is that the other gases are better absorbers of sunlight, but CO2 is a better absorbed of terrestrial IR. Does this mean that you believe that the effect of adding CO2 would be a negative effect on temperature in the day and a positive effect at night?
> While the experiment isn't ever going to properly reproduce the mechanisms that result from higher CO2 in the atmosphere, as a measure of CO2 absorbtion of IR, the experiment would have a better chance if conducted at night with the thermometers under the bags.
Can you please explain why the effect would be different with the CO2 in the atmosphere as opposed to in the bag? Is there more to it than simply more CO2 content in the air in a specific location? Are there additional macro-effects whe the CO2 is in the atmosphere that the bag experiment would not be able to emulate?
Also, please clarify "under the bag", not "in the bag"?
Bob:
Would the fact that both thermometers are enclosed in the bag act as a control?
If there is a difference to the thermometer reading itself, due to it's being enclosed in CO2 rather than air, how significant do you think that would be? I.e. How many degrees influence?
Also, if there is an influence, isn't it highly unlikley that it would be exactly equal to the influence that the CO2 is having on warming the bag, resulting in a net zero reading?
Thanks for taking the time to return your answers.
Regards,
Jessica
P.S. If someone caould confirm what the expected temperature change per increased unit of ppm of CO2 (or "doubling of CO2") is, that would be great. Thankyou.
Hi MA,
FYI, I am repeating the study again, at night, as you suggested. So far there is no noticeable difference.
Thanks for the advice,
Jessica
Hi Glenn,
Thanks for your response.
>When the CO2 is released from the spritzer, the gas will come out somewhat cooled, so that may explain the lower CO2 temperature in the bag. How good an insulator is the plastic, that may impact how easily any temperature difference due to this corrects?
The temperature did drop immediately (by about 1 degree) as you suggested as the CO2 comes out colder. I monitored the two bags for probably an hour or so so I imagine that would have corrected - also, when I took them outside, the temperatures of each rose by about 5 degrees, so I assume this would not have happened if the bags were good insulators.
>Next, although CO2 absorbs infrared, most of that absorption in the atmosphere takes 100's to 1000's of meters to be 100%. How much will be absorbed over inches?
Do you mean that it takes 100s to 1000s of meters at present CO2 levels to absorb all of the radiation available to it? I don't think this should matter. I am simply increasing the amount of CO2 in one particular point which will have a given amount of radiation, this should theoretically have a temparature effect (if the mechanism is as simple as add more CO2, absorb more radiation, get more heat).
> Next, what is the transmissivity of the plastic - how much radiation passes through the bag? The bags may be transparent to visible light (very high transmissivity) but most materials behave very differently for infrared light. Most naturally occuring materials have extremely low transmissivity to infrared. You would need to research the properties of the plastic involved. Otherwise you are effectively carrying out the experiment with an opaque (to infrared) bag.
Here is the infrared spectrum of polyethylene:
https://azom.com/images/Article_Images/ImageForArticle_12386(1).jpg
As you can see, here:
https://webbook.nist.gov/cgi/cbook.cgi?Spec=C124389&Index=1&Type=IR
The infrared spectrum of CO2 is different, there may be some overlap, but the bulk of what CO2 absorbs should pass thorugh the polyethylene plastic.
> The greenhouse effect depends on the behaviour of the entiure atmosphere over vertical distances of many kilometers. Extra absorption by CO2 at the surface is only a small part of any change in the GH effect. The big changes involve how much emission by CO2 changes at high altitude - 10 km or so. It is very hard to model the GH effect with small, surface based setups.
I can see that adding more CO2 to the atmosphere would have an overall effect if CO2 causes temperature to change. For example, if CO2's primary source of heat-producing radiation is terrestrial IR, and you add more CO2 to the atmosphere generally, the CO2 might absorb more of the IR closer to the Earth's surface, leaving less to be absorbed at higher altitudes, so temperatures might be warmer at low altitudes and cooler at high altitudes (at, say, 10 km up). But this is a prediction of how the atmosphere would respond given that CO2 has this effect. It's jumping the gun because I'm trying to prove the effect to begin with. I don't see why a pocket of air with more CO2 (as in the experiment) would not generate any noticable heat difference. The radiation available to it is the same, you are simply adding more CO2, which should theoretically result in a higher temperature locally (just as if you added CO2 at any particular point in the atmosphere, you should generate a higher temperature locally).
Thanks for your response,
Jessica
jesscars @337,
CO2's main absorption wave-length is at 12 microns. This is at the peak of the Earth's IR emissions but is an irrelevant part of sunlight. Thus a High-CO2 bag would be pretty-much transparent to sunlight as well as to the Earth's IR except at that 12 microns wavelength. This transperancy is because in the bag world, the CO2 has purged all the other GHGs and thus reducing the warming from those other GHGs. This one-step-forward-one-step-back doesn't happen under AGW. Indeed, a CO2-warmed world results in elevated water vapour, another GHG. AGW is operating day & night but the night-time temperatures will be boosted more than the day-time ones.
The actions of CO2 as a GHG is not that of a simple insulation layer (although the circulations in the atmosphere are far less leaky than the leaks in any greenhouse). One (simplistic) mode of warming concerns the altitude at which the IR at 12 microns can get a clear-shot out into space. As more CO2 enters the atmosphere, the clear-shot altitude increases and the temperature at that clear-shot altitude drops. The lower the temperature, the less IR energy the clear-shot gases can shoot out into space. The bag world cannot demonstrate such AGW mechanisms.
One mechanism (the significnt one, we are told) concerns the breadth of the 12-micron band. At the flanks, only whizzy-whizzing CO2 molecules can catch the IR and as CO2 levels increase, so the whizzy-whizzing CO2 becomes denser & the flanks expand. This is the logorithmic 1ºC of warming for double CO2 (without feedbacks). With the High-CO2 bag, there are perhaps 10X doublings? and if we run with this back-of-fag-packet calculation, that would suggest the High-CO2 bag would see 10X 1ºC warming while the Atmosphere bag would have the planet's GHG warming which is usually reckoned to be about 30ºC. This suggests that the extra warming from the High-CO2 bag would not compensate for the GHG warming purged relative to the Atmosphere bag. Mind, the humidity will play a big big part in any such calculation.
And under the bag? was simply to mirror the atmosphere being above the AGW-warmed surface. In the bag? would do as well if the position of the thermometer can be precise.
Hope this blather has addressed your queries.
I would emphasis what others are saying here:
1/ what you are trying to do is demonstrating the radiative properties of CO2 which is central to the GHE, but you cannot demonstrate the GHE in a simple column of gas.
2/ Getting the experimental setup right is difficult as it is easy to overwhelm the CO2 radiative effect with other spurious influence. Have a look at this setup to make a better attempt.
Spend some time looking at the basic mechanics of how the GHE really works. Eg here or Science of Doom It's a lot more subtle in the full mechanism than a naive approach would expect. I would throughly recommend Wearts history of discovery of the global warming for its insight into the how experiments have helped and hindered the development of the science.
Another point about Tristan/jesscars experiment that has only occurred to me reading the later comments. In the orignal description, the comparison is between a bag filled with normal air, and a bag filled with CO2.
As water vapour is also a greenhouse gas, the comparison is actually between two bags filled with different amounts of two different IR-absorbing gases. My first guess is that the CO2 would be a considerably greater IR absorber, due to its greater concentration, but You'd have to do the math. It does illustrate that there are a lot of fine details that need to be tracked.
In experimental design, you really have to make sure that the variable of interest is indeed the main variable.
Hi MA,
Thanks for the response.
> CO2's main absorption wave-length is at 12 microns. This is at the peak of the Earth's IR emissions but is an irrelevant part of sunlight. Thus a High-CO2 bag would be pretty-much transparent to sunlight as well as to the Earth's IR except at that 12 microns wavelength. This transperancy is because in the bag world, the CO2 has purged all the other GHGs and thus reducing the warming from those other GHGs. This one-step-forward-one-step-back doesn't happen under AGW. Indeed, a CO2-warmed world results in elevated water vapour, another GHG. AGW is operating day & night but the night-time temperatures will be boosted more than the day-time ones.
I can agree that the bag purges the other gases, so doesn't experience warming from those gases, but if they have a higher heat-producing capacity than the CO2 that replaces them, why would the net effect of adding more CO2 to air be to increase temperatures? I repeated the same experiment at night (indoors) and again, there was no notcieable difference in temperatures.
> The actions of CO2 as a GHG is not that of a simple insulation layer (although the circulations in the atmosphere are far less leaky than the leaks in any greenhouse). One (simplistic) mode of warming concerns the altitude at which the IR at 12 microns can get a clear-shot out into space. As more CO2 enters the atmosphere, the clear-shot altitude increases and the temperature at that clear-shot altitude drops. The lower the temperature, the less IR energy the clear-shot gases can shoot out into space. The bag world cannot demonstrate such AGW mechanisms.
I agree with this too, that if CO2 has a warming effect, the effects of CO2 in the bag do not represent what will happen in the atmosphere. But why wouldn't the bag show any warming at all? I don't see why adding more CO2 to the atmosphere should effect temperatures, but adding CO2 to the bag has no effect on temperature. If there is a global or atmospheric effect of adding CO2, why isn't there a local one (in the bag)?
Hi Bob,
Thanks for your response:
> The air-filled bag would have water vapour in it - in whatever amount is present in the air at that time. (Probably at most a few percent.)
The CO2-filled bag would be dry - i.e. no water vapour.
As water vapour is also a greenhouse gas, the comparison is actually between two bags filled with different amounts of two different IR-absorbing gases. My first guess is that the CO2 would be a considerably greater IR absorber, due to its greater concentration, but You'd have to do the math. It does illustrate that there are a lot of fine details that need to be tracked.
H20 appears to be a more effective GHG by comparing at the infrared spectrums, here and here. The CO2 filled bag would have approx 1/4 the amount of normal air. I suppose I would have to do the maths, though I would have to know how effective the additional CO2 is and how effective the H2O is at creating heat. It does seem strange though that there is no real noticeable difference in the temperatures either indoors or outdoors or at night - it seems unlikely that these would always balance out.
Hi scaddenp,
Thanks for the response.
> 1/ what you are trying to do is demonstrating the radiative properties of CO2 which is central to the GHE, but you cannot demonstrate the GHE in a simple column of gas.
Why not? I am not sure why the warming effect of CO2 would not be seen in a local pocket of high-CO2 air.
> 2/ Getting the experimental setup right is difficult as it is easy to overwhelm the CO2 radiative effect with other spurious influence. Have a look at this setup to make a better attempt.
Thanks for the comment. I have seen a similar experiment done on youtube, but my problem is that CO2 is what's being added to the atmosphere, there is no additional radiation other than what is there naturally. So using a heat lamp, unless it emulates only natural radiation variations does not a represent what will happen when CO2 is in the natural atmosphere.
Why not?
Perhaps you should tell us how you think the GHE works? Check those references. An isothermal column will not have a GHE.
Any scientific experiment depends on controlling the variables in the experiment. As has been pointed out to you, your design has all sorts of issues with that. If you want to do this outside, then you need to do it at night really. The radiation that the CO2 is interacting with (IR) comes from heat re-radiating from the surface and that being re-radiated from GHG in the atmosphere all the way up. Not easy to control. Direct measurement of the GHE in the atmosphere is a complex experimental design, not for amateurs. See this paper for instance on how to really do it.
Using heat lamp as proxy for IR being irradiated surface allows you some control and at level which makes it measurable.
Hi All,
I have another question re. temperature predictions.
If the expected warming is an increase of 1 degree per doubling of CO2, why is this not matched by the Vostok Ice Core samples? These show about a 1 degree per 10 ppm linear relationship.
Why would the historic linear trend be replaced by a logarithmic one? At what level of CO2 does this happen?
Thanks,
Jessica
Hi scaddenp,
My understanding is that CO2 molecules absorb terrestrial IR then reradiates it as heat. I'm not sure why it should matter if that's a bag with a higher concentration of CO2 or CO2 molecules in the atmosphere. I don't see why there should be a difference. Are you able to explain this to me? I will also check the papers.
OK, so you acknowledge that the warming, when under natural sources of radiation is insignificant. I understand that adding radiaton would increase temperatures, but the GHG of the atmosphere will only ever face natural sources so it doesn't represent what will ever actually happen.
I have tried the experiment at night, indoors, and outdoors, and with differnt concentrations of CO2. There is never a significant difference - definitley not 1 degree per doubling.
Also, can you please confirm the 1 degree per doubling and where this comes from? This is not matched on the Vostok Ice Core samples, which show a linear relationship of about 1 degree per 10 ppm. I am not sure why the discrepancy in the science.
Thanks,
Jessica
jesscars @343,
The relative strength of CO2 as a GHG is dependent on the logarithmic nature of its forcing. The first doubling will, molecule for molecule, be twice as 'forceful' as the second doubling and a thousand times more 'forceful' than the tenth doubling. So the 'forcefulness' you measure in the High-CO2 bag will be mainly a thousand-times weaker than the CO2 'forcefulness' involved in AGW. And while the ten doublings of CO2 together will provide a very 'forceful' GHG effect at 15 microns, (By-the-way, I note my 12 microns @340 is wrong - it is 15 microns.) this is achieved by stripping all GHG from everywhere else. This one-step-warmer-one-step-cooler effect for the bag world could well explain the non-result although there could be many other contributing reasons.
jesscars @347,
Your comparison of the 1ºC of warming for double CO2 (without feedbacks) with the Vostok Ice Core temperature/CO2 graph doesn't properly hold. Firstly, the Vostok temperatures will be subject to polar amplification and Ice Ages result from other non-CO2 'forcings' (CH4, ice albedo) and their feedbacks. The direct CO2 contribution (without feedbacks) to the Ice Age cycles (which are globally some 5ºC) is probably something like 0.5ºC, which fits in with the logarithmic relationship. With feedbacks, the CO2 'forcing' is responsible for about a third of the Ice Age wobbles.
The logarithmic nature of CO2 forcing holds certainly for 180ppm to 2,000ppm. (See for instance Etminan et al 2016.) At very low concentrations it will presumably be more linear (like CH4) but the point of change from logarithmic is not something I have met. Persumably the level is well below any CO2 levels ever seen on Earth.
Jesscars — as an average reader here, I am puzzled why you persevere in trying to "fix" your backyard experiment set-up intended to replicate the already-demonstrated empirical evidence of CO2 "greenhouse".
(A) Firstly, there is the empirical evidence from experimentation during the past 150+ years, showing CO2 absorption of (some) Infra-Red radiation. (B) During the past century there is the empirical evidence of the CO2-related global Green House Effect [GHE] : evidence provided by both expensive and (relatively) cheap experimentations & observations. (Admittedly, "greenhouse" is a poorly-named term — but historically we are now stuck with it, and it is now a widely-understood useful label.)
Your experiment is inappropriate because of its lack of sensitivity and specificity (too many confounding variables in your experimental set-up).
Not only do you need to address the question of IR absorption by CO2 gas, but secondly you need to address the mechanism of the planetary GHE (a mechanism which is completely unconducive to backyard experimentation, I think).
And your later questions indicate that you have not grasped the essentials of global-scale surface temperature changes. Climate is a complex matter, and you must not expect to understand all the science of it, by means of a few paragraphs of explanations — You owe it to yourself to undertake basic self-education by extensive reading (and/or by some of the excellent video-tutorials available. And if you want to be entertained humorously by videos while self-educating, then seek out the series of Potholer54 videos — they educate indirectly, by amusingly debunking the numerous scientific errors committed by the anti-science brigade i.e. denialists. Potholer54 also does a series on evolution-deniers . . . but you probably won't have time for that sort of humorous entertainment sideline.)
The more you learn, the better you will be able to ask appropriate questions. Start from the basics, and then you can usefully re-visit Vostok and global warming response curves. As MA Rodger has implied, you have been trying to put the cart before the horse.