Recent Comments
Prev 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Next
Comments 151 to 200:
-
Bob Loblaw at 06:03 AM on 29 November 2024CO2 effect is saturated
Charlie_Brown @ 787:
CallItAsItIs has not shown much proclivity for following links and reading material people have pointed him to. (He doesn't even seem to read much of what people say in comments to this thread.)
Anyway, more to the point, since figure 3 in your guest post is a figure that is "available on the internet", you can easily re-use that figure and insert it into a comment, just like any other figure that is "available on the internet". I did this earlier for the Trenberth diagram, which is used on another SkS page.
The link to your figure is:
https://skepticalscience.com/pics/AtmosphericRadiationModel-Fig3-1200px.jpg
...and I can insert it here using the little "tree" icon on the Insert tab of the editor:
(In this case, I used the Appearance tab on the "insert Image"dialog box so that I could limit the display width to 500 pixels. This avoids breaking the web page formatting with large pictures.)
-
Charlie_Brown at 04:32 AM on 29 November 2024CO2 effect is saturated
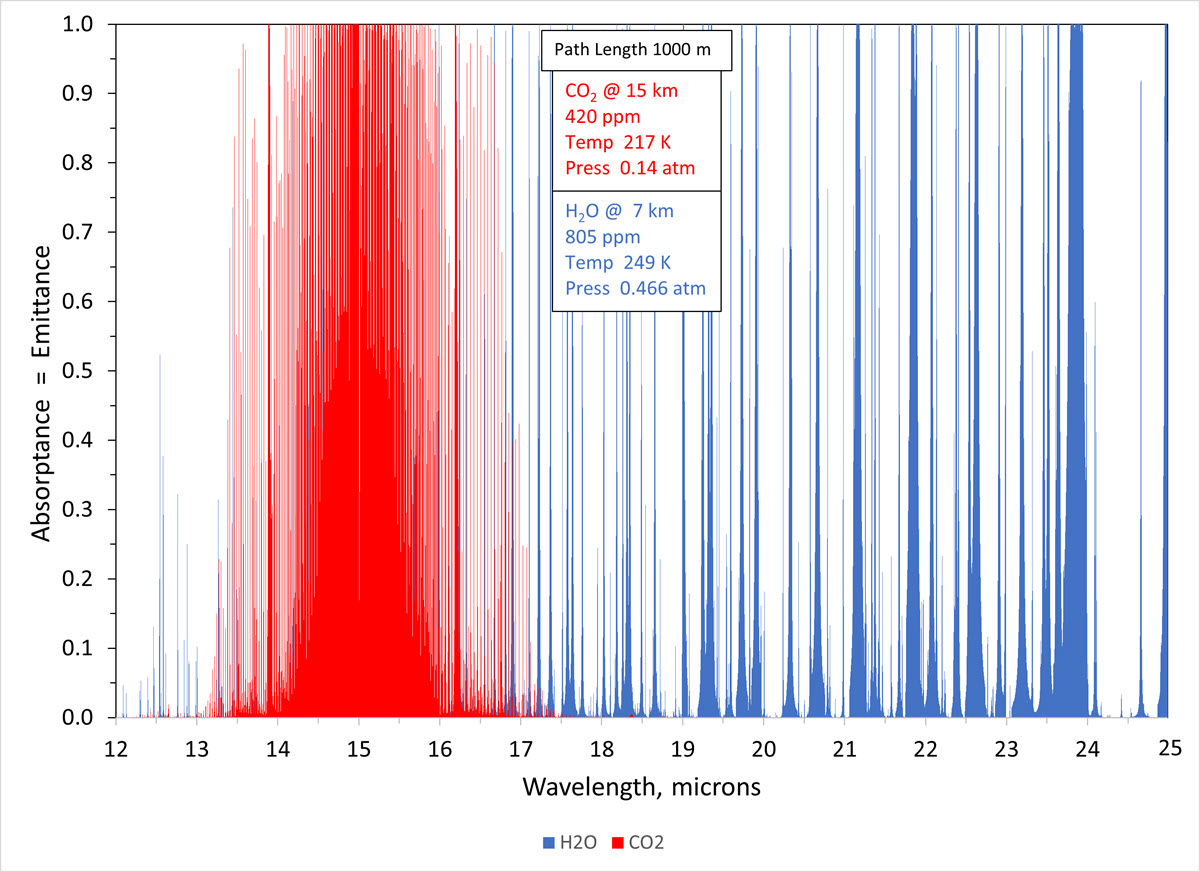
A key point that explains why the CO2 band is not saturated has been lost in trying to convey fundamental concepts. To avoid semantics, I use the term “saturated” to mean no further effect from increasing CO2. This occurs when emittance = maximum value of 1.0. It should have been evident in Figure 3 of the link to my guest post that Bob provided earlier. It also should have been apparent when I said that the 15-micron peak at 50 km was below the Planck distribution temperature. The emittance of the strongest lines between 14.93-15.0 microns absorptance/emittance lines reach a value of 1.0 in the lower atmosphere and at the bottom of the stratosphere. However, the CO2 band has thousands of strong and weak emittance lines between 13-17 microns. The emittance of the 14.25-micron line has a value of 0.25 in the tropopause where it matters to energy loss to space. By Beer’s Law, the line will strengthen with increasing CO2. The 14.93-15.0 micron band may be saturated with respect to energy loss to space. However, the full range of the CO2 band is not saturated. The logarithmic band saturation effect is plotted in Figure 3 of my article in Chemical Engineering Progress that was referenced in the guest post.
CallItAsItIs, as a professor for an in-person class, I do not respond well when a student stands up and shouts “Wrong!” (@760), disrupts the class, and then repeats his misunderstanding. In person, that student would be dismissed. Let me know, with some respect, if you have any further questions.
-
Bob Loblaw at 00:36 AM on 29 November 2024CO2 effect is saturated
CallitAsItIs @ 782:
You say (your emphasis):
Kirchhoff’s Law is absorptance = emittance (at thermal equilibrium)
Actually, its thermodynamic equilibrium.
You go on to say (emphasis mine):
Now, the system is never far from equilibrium, but laws that are strongly conditioned on equilibrium may well be compromised.
Translation: CallItAsItIs feels free to invoke Kirchoff's Law when he wants to, and ignore it when it is inconvenient to his bogus argument.
Let's look at a "radiometry textbook", since CallItAsItIs is so fond of them. I'll go back to Liu (1980) "An Introduction to Atmospheric Radiation", which I mentioned earlier as one of the books on my bookshelf. On page 13:
Kirchoff's law requires the condition of thermodynamic equilibrium, such that uniform temperature and isotropic radiation are achieved. Obviously, the radiation field of the earth's atmosphere as a whole is not isotropic and its temperatures are not uniform. However, in a localized volume below about 40km, to a good approximation, it may be considered to be isotropic with a uniform temperature in which energy transitions are determined by molecular collisions. It is in the context of this local thermodynamic equilibrium that Kirchoff's law is applicable to the atmosphere.
If CallItAsItIs wants to continue to ignore Kirchoff's law when forming his arguments, he will need to provide a much stronger argument as to why it does not apply than to hand-wave it away with a statement such as "may well be compromised".
-
MA Rodger at 00:26 AM on 29 November 2024CO2 effect is saturated
Through the last 50-odd comments it is evident that the commenter CallItAsItIs is unable to accept that poly-atomic molecules in a gas will pick up vibrations from collision, this often enough that some of them will relax and emit a photon before further molecular collision. He seems to still believe that ideal gases exist. SO that's quite a pile of learning he would need to grasp to understand the greenhouse effect in terms of the molecular processes.
-
Bob Loblaw at 00:18 AM on 29 November 2024CO2 effect is saturated
CallItAsItIs @ 781:
Finally, we get to see something from you that is a little closer to reality, but once again you wander into interpretations that are blatantly wrong. Let's start with part of your second paragraph:
An energized CO2 molecule could also re-emit a similar photon in the downward direction, and this new photon is re-absorbed by the earth, although this is far less likely. Photons absorbed and then re-emitted in an upward direction would not be distinguishable from those not absorbed in the first place.
First of all, emission of IR radiation by CO2 (or any other gas in the atmosphere) is omni-directional. Up. Down. Left. Right. North. South. Southwest. All directions are equally probable. As a result, IR radiation is diffuse in nature - emission heads out in the shape of a sphere. It is convenient to consider this spherical problem in the context of two directions - a hemisphere we'll call "up" and a hemisphere we'll call "down". This leads to a well-known (for those of us informed on atmospheric radiation transfer) analysis named the two-stream approximation.
The key result is that at any height in the atmosphere, an IR photon emitted by CO2 has an equal probability of being emitted upward or downward. And yes, this "new" IR photon is indistinguishable from any other IR photon of the same wavelength. As such, it is just as easily absorbed as photons originating from the surface. In fact, we can calculate the probability of that absorption by applying - wait for it - Beer's Law, using the height of emission as the starting point and following the path through the atmosphere. If we want to include the emission of radiation and work it in with Beer's Law to get a more complete equation, we can get the Schwarzschild’s equation.
Way back when, you argued that CO2 in the atmosphere above 10m could not possibly absorb 15um photons because there weren't any left is now shown (by your own admission) to be completely wrong. There is a ready supply of 15um photons available, travelling in all directions. (You still vastly underestimate how many there are, and have missed out on the ones that are heading upward, but it's a start.) Earlier, you dismissed those upward-directed 15um photons as "thermal radiation" not related to CO2 - hopefully now you realize why we've been telling you that you are wrong. [But your paragraph 4 reduces that hope. See below.]
On to paragraph 3, which starts:
When I made the statement that conservation of energy must hold for each frequency independently of the others, I was merely trying to provide the physical basis for applying Beer's Law to individual frequencies. But since you are convinced of that already, you can disregard this statement.
Frankly, that is bull$#!^. Conservation of energy has nothing to do with applying Beer's Law to individual frequencies. And no, we will not disregard that statement because it is yet another example of where you clearly do not understand the physics involved. Invoking an incorrect, imaginary explanation only serves to destroy your credibility.
Paragraph 4:
Now that we know that Beer's Law applies to the 15 micron absorption band, we see that this band is attenuated to insignificant values well below the TOA, with the electromagnetic energy being converted into thermal energy mostly within the atmosphere. Furthermore, adding more CO2 won't cause more warming because this band is already completely absorbed and converted into heat.
...and you are back to completely ignoring the observed fact that 15um radiation does exist in the upper atmosphere (moving in both upward and downward directions) in highly-significant values, and that CO2 can absorb these 15um photons (since you admit that these 15um photons are indistinguishable from ones emitted at the surface). And when you do the full math using the proper equations for absorption, emission, and conservation of energy, you find that adding CO2 does cause more warming.
-
Eclectic at 17:41 PM on 28 November 2024CO2 effect is saturated
CallItAsItIs :-
Absolutely no need to apologize for an "almost" double posting. After all, it did provide opportunity for a tad of humor.
[ If you happen to double-post in future, then simply do an additional brief post asking the Moderator to make one deletion at his early convenience. ]
Also ~ while our friend Michael Sweet is skeptical about your bona fides as a genuine poster . . . that is ultimately a matter for an umpire's decision by Moderators, who may (or may not) expunge the posts of clumsier trolls or of those whose crackpot ideas are expressed in a repetitive tiresome manner.
Some crackpots simply do a "drive-by" series of posts on SkS and then disappear ~ others will later phoenix themselves. But we hope they will then revise & renovate their contrarian arguments into something of at least 25% merit. # Sadly, I have never encountered a phoenix who manages to show that all the mainstream scientists are wrong. Yet we live in hope that AGW will someday prove to be a mistaken concept, eh.
I am still eating my popcorn, CallItAsItIs , while I wait for you to introspect about Motivated Reasoning. And I suspect that some of the reason you are here in this thread, is that the strictly rational part of your intellect is quietly hoping that the powerful emotional/non-rational part of you will be brought into agreement with the climate expert physicists. ( Ain't human psychology interesting ! )
Moderator Response:[PS] This thread is on the usual downward spiral. CallItAsItIs you are ducking and avoiding the germaine questions and still apparently refuse to read refutations or clarifications. It is time put up or shut up. Ramanathan and Coakley 1978 https://ramanathan.ucsd.edu/wp-content/uploads/sites/460/2017/10/pr15.pdf put a clear early picture of solar flux. Since you strongly disagree, please give us your equations for describing the energy flux through a layer in atmosphere. Then we can compare with observations and decide scientifically.
-
CallItAsItIs at 15:03 PM on 28 November 2024CO2 effect is saturated
There is something concerning Kirchoff's Law that we all may have forgotten about. Actually, Charlie_Brown brought it up at 756.
Kirchhoff’s Law is absorptance = emittance (at thermal equilibrium) (emphasis added)
At this point, it must be remembered that a system in the middle of absorbing photons is not in thermal equilibrium since more energy is being added. After absorption, the newly introduced energy is re-distributed throughout the system (the atmosphere in our case) and IR spectrum in order to re-establish equilibrium. Now, the system is never far from equilibrium, but laws that are strongly conditioned on equilibrium may well be compromised.
-
CallItAsItIs at 14:22 PM on 28 November 2024CO2 effect is saturated
Eclectic @779
First, I would like to let you know that my posting very nearly the same comments twice was unintentional. I submitted the first one but it didn't show up on the webpage. So, I figured that somehow it got lost and I rewrote it and re-posted it. But it seemed to get "lost" again. Finally, I realized that I had started a new page with my new comment. So please disregard one of those postings and your moderator is welcome to remove it.
Next, the reason I "failed" to include where the absorbed photon energy goes is because I thought we already knew that. Primarily, it is distributed as kinetic energy to the atmospheric gases through collisions of excited CO2 molecules with N2 or O2. An energized CO2 molecule could also re-emit a similar photon in the downward direction, and this new photon is re-absorbed by the earth, although this is far less likely. Photons absorbed and then re-emitted in an upward direction would not be distinguishable from those not absorbed in the first place.
When I made the statement that conservation of energy must hold for each frequency independently of the others, I was merely trying to provide the physical basis for applying Beer's Law to individual frequencies. But since you are convinced of that already, you can disregard this statement. This means that the energy flux contained in each frequency is proportional to the amplitude squared of the electric (or magnetic) field at that frequency, and the energy flux of the entire spectrum is simply the sum of the energy fluxes at the individual frequencies. This, of course, should be nothing new to persons knowledgeable in radiometry.
Now that we know that Beer's Law applies to the 15 micron absorption band, we see that this band is attenuated to insignificant values well below the TOA, with the electromagnetic energy being converted into thermal energy mostly within the atmosphere. Furthermore, adding more CO2 won't cause more warming because this band is already completely absorbed and converted into heat.
At this point, it looks like you have a few things you could mull over yourself from your comfortable chair and popcorn.
-
michael sweet at 13:30 PM on 28 November 2024CO2 effect is saturated
Bob and Eclectic
Callitasitis strongly reminds me of a user who has been banned repeatedly. I doubt that you will be able to explain thermodynamics to them no matter what you post.
Keep in mind that the comments policy doesn't allow repetition. When Callitasitis repeats themselves repeatedly it is time to let other readers decide who has presented the better arguments.
-
Eclectic at 06:51 AM on 28 November 2024CO2 effect is saturated
CallItAsItIs @776 and @777 :-
Curiouser and curiouser . . . you make two almost identical posts, just 16 minutes apart. Can I believe the evidence of my "linear optics"? (Please excuse my humorously lame misquote from a certain third party.)
And in post @778, you see another "Law" (i.e. the LobLaw of Unintended Consequences ).
Also, CallItAsItIs, you state (twice) that: "I should also point out I am not using any tricks not already used by your AGW believing comrades." [unquote, unquote]
And there we touch upon the heart of your problem. Motivated reasoning. Motivated Reasoning does produce ~ even in an intelligent person such as yourself ~ the most remarkable contortions of self-contradictory assertions. As we have seen.
CallItAsItIs, you have a great deal of your own statements to reconcile. In the meantime, I shall get me some more popcorn, and a comfortable chair.
-
Bob Loblaw at 06:40 AM on 28 November 2024CO2 effect is saturated
Congratulations, CallItAsItIs. You actually have some sort of access to a textbook that covers "radiometery", and you know how to look in an index.
I am familiar with Petty's book, although It is not one that I have on my personal bookshelves.
I'll see your Petty, and raise you a Wallace and Hobbs, "Atmospheric Science, an Introductory Survey" (Beer's Law discussed on pages 296-297), a Pierrehumbert "Principles of Planetary Climate" (Beer's Law discussed in chapters 4 and 5), a Liou "An Introduction to Atmospheric Radiation" (which has 6 sections listed in the index for the Beer-Bouguer-Lambert law), and an Oke "Boundary Layer Climates" (also multiple references in the index).
All of those four books are ones that I do have on my personal bookshelf.
And if you want to see what else I know about Beer's Law, you can read this post:
https://skepticalscience.com/from-email-bag-beer-lambert.html
Yes, Beer's Law applies to individual wavelengths/frequencies. I challenge you to find a single reference that supports your argument that "conservation of energy must hold for each frequency independently of the others."
You see, Beer's Law says nothing at all as to what happens to energy that is absorbed when photons disappear within the volume of air it includes. As far as Beer's Law is concerned, the energy simply disappears along with the photon. To apply "conservation of energy" principles, you need to include where that energy goes - which you repeatedly fail to do.
-
CallItAsItIs at 05:41 AM on 28 November 2024CO2 effect is saturated
Bob Loblaw @775Sure! Try A First Course in Atmospheric Radiation, 2nd edition, by Grant W. Petty. He first gets into Beer's Law at page 78. I should warn you, however, that this book assumes you already know that since we are dealing strictly with linear optics, it is perfectly legitimate to break down the total EMR into individual frequencies, and consider each one as independent of the others. If this gives you heartburn, then I suggest you study up on some basic E&M.
I should also point out that I am not using any tricks not already used by your AGW believing comrades.
-
CallItAsItIs at 05:25 AM on 28 November 2024CO2 effect is saturated
Bob Loblaw @775
Sure! Try A First Course in Atmospheric Radiation, 2nd edition, by Grant W. Petty. He first gets into Beer's Law at page 78. I should warn you, however, this book does assume that you already recognize that it is perfectly legal to consider the EMR one frequency at a time since we are dealing strictly with linear equations. If this gives you heartburn, I might suggest you first study some basic E&M and differential equations.
I should also point out that I am not using any tricks that are not already used by you and your AGW believing comrades.
-
Bob Loblaw at 00:55 AM on 28 November 2024CO2 effect is saturated
One more "one more". CallItAsItIs states in 769:
...please understand that I cannot pack an entire radiometry textbook into this comment space.
I challenge you to actually name just one "radiometry textbook" that you have read. Bonus points if you can point to a section of such a book that supports any of your postings here.
-
Bob Loblaw at 00:52 AM on 28 November 2024CO2 effect is saturated
One more. CallItAsItIs says in comment 769:
Because when we break down the EMR into the sum of contributions from the different frequencies it contain, we find that each such contribution is incoherent relative to the others.
The only things that is incoherent is CallItAsItIs's physics. Absorption and emission of radiation are independent events. Once again, I beg that CallItAsItIs read Eli Rabbet's blog post on the time scales involved in absorption, emission, and collisions with other molecules. [CallItAsItIs: the previous sentence includes the link to that blog post.]
To start, here is the opening section of Eli's post:
One of the useful things the Rabett used to do was to explain what happens to the energy when a molecule, say CO2 (carbon dioxide) although you could also say H2O (water vapor) or CH4 (methane) absorbs light. For the purpose of this post, the photon would be in the infrared region of the spectrum. This is an evergreen for two classes of bunnies
- Bunnies who don't realize that the molecule can also emit light. This is a popular one amongst organikers and analytical chemists whose experience with IR spectroscopy is in an absorption spectrum for analysis of samples
- Bunnies who think that the only way that an excited molecule can get rid of the energy is to emit a photon.
I will leave it as an exercise for the reader to decide whether CallItAsItIs falls into class 1, class 2, or both.
-
Bob Loblaw at 00:41 AM on 28 November 2024CO2 effect is saturated
CallIiAsItIs @ 766:
I need to quote this in its entirety for context. Your comment says:
Bob Loblaw @762
The catch is that all CO2 molecules are continually emitting radiation. This represents an energy loss. And they make up for that energy loss by colliding with other molecules (such as N2 and O2) and gaining energy when those molecules have higher energy.
And how do they get this extra energy? — from Maxwell's Demons! (LOL)
I assume that you mean "other molecules" when you say "they'. Well, if you read my comment at 772 (and all of my comment at 762) and look closely at the Trenberth diagram, you will discover that the other molecules can get that energy from absorbing IR radiation, visible light, UV radiation, thermal transfers from the surface, evaporation from the surface, or simply by colliding with yet more molecules that have received energy from any of those sources.
Unfortunately, I don't think that any of that is going to sink in for you, since it becoming abundantly clear that you have an extremely strong Morton's Demon filtering your "knowledge" of physics.
-
Bob Loblaw at 00:32 AM on 28 November 2024CO2 effect is saturated
CallitItAsItIs @ 765 (where he responds to my request for his definition of "sources of energy"):
You are in no position to tell other people to "learn some physics". Let's start with one of your statements:
...we are trying to determine the warming of the atmosphere due to GHGs tapping energy from the terrestrial IR radiation rising from the surface. This means that the upwelling terrestrial IR radiation is the source.
Once again, you are wrong. Let's look at Trenberth's diagram again:
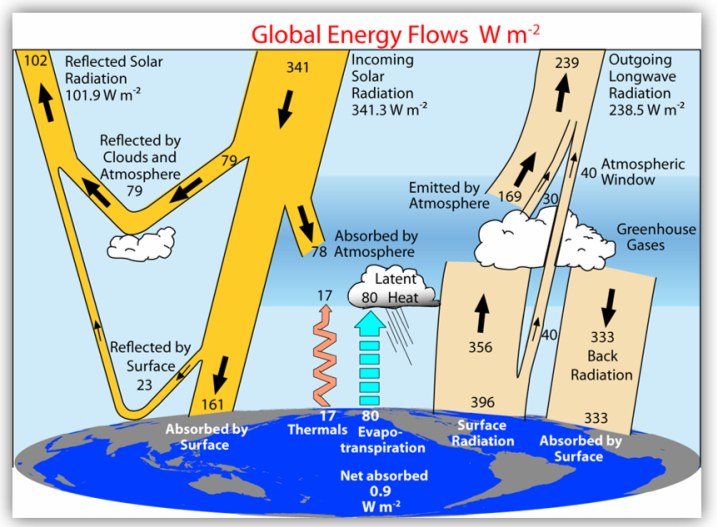
You clearly have no idea what this diagram shows. I will point specifically to two arrows in the middle of the diagram, originating at the surface. The ones labelled "Thermals" and "Evapotranspiration". Those are flows of energy from the surface ("source") to the atmosphere (sink, if you like). IR radiation (labelled "Surface radiation") is to the right, and it is not the only transfer of energy from the surface to the atmosphere.
You continue with:
The sun also is a source of energy since it puts out IR radiation which is absorbed by the GHGs and converted into thermal energy in the same manner as the terrestrial IR radiation.
Once again, you ignore anything other than IR radiation. A lot of the sun's direct warming of the atmosphere comes from absorbing non-IR radiation - visible light, and UV radiation. In fact, the main reason that the stratosphere is much warmer than the troposphere is because of UV absorption by ozone. The atmosphere is not completely transparent to visible or UV radiation.
Then you state (with respect to surface heating):
The down-welling terrestrail radiation from the atmosphere is another a source, but a much weaker one.
Look at the Trenberth diagram again. Solar radiation absorbed by the surface is 161 W/m2. (On the left side.) If you look on the right side, you see that "Back Radiation" (IR from the atmosphere to the surface) is 333 W/m2. I challenge you to find one reputable source that says 333 is "much weaker" than 161.
..and if you look closely at the IR radiation flows between the surface and the atmosphere (on the right of the diagram), you will see that the net exchange is only +23 W/m2 - the atmosphere only absorbs 356 W/m2 of the 396 W/m2 coming off the surface, but sends 333 W/m2 back to the surface. Contrast that with the 97 W/m2 (17+80) transferred from the surface to the atmosphere by thermals and evapotranspiration, and add in the 78 W/m2 of solar radiation absorbed directly by the atmosphere (in the middle of the diagram) and you get a total of 175 W/m2 of energy added to the atmosphere from sources that are not surface emission of IR radiation.
And then in your closing paragraph, you state (emphasis added):
Since the contributions to the total upwelling EMR at different frequencies involve different photons, conservation of energy must hold for each frequency independently of the others.
And this is probably the root cause of your confusion. No, conservation of energy is not something that must hold for each frequency independent of others.
Once CO2 (or any other material) absorbs a photon, the energy gets transformed into another form (thermal/kinetic, chemical, etc.) and the CO2 is free to do whatever it wants to (restricted by physics and chemistry, of course) with that energy. It can emit it as radiation in any frequency of the many it is capable of absorbing or emitting. It can keep it as kinetic (thermal) energy. It can dump it off as kinetic energy to other molecules it collides with as it bounces around in the sky.
The energy contained within the CO2 molecule has no memory of where it came from. Absorption of radiation, kinetic transfer from colliding with other molecules, etc. It's all just energy once it is stored in the molecular structure of the CO2.
Energy conservation only applies to the system as a whole. Your version of "physics" is bordering on crackpot territory.
-
Eclectic at 21:55 PM on 27 November 2024CO2 effect is saturated
CallItAsItIs @770 :-
If I may answer on Scaddenp's behalf ~ I would point out that what you say could have relevance on Mars with a pure CO2 atmosphere (disregarding a trace of nitrogen & argon). But on planet Earth, your explanation clearly fails the reality test.
*
CallItAsItIs @769 :-
Well, I give you points for being entertaining !
My cynical streak impels me to the suspicion that you are using AI-generated language to produce a garbled incoherence of "explanations".
Even better is your novel suggestion that atmospheric molecules cannot give or receive energy to/from other atmospheric molecules (why ~ because Second Law of T , of course, eh what, eh ).
And surely only an AI would assert that H2O molecules have no indirect or direct effect or interaction (kinetic or photonic) with nearby N2 , O2 , or CO2 molecules.
Vraiment une tour de force, mon ami. (Does the AI tackle French, as well? )
-
CallItAsItIs at 21:01 PM on 27 November 2024CO2 effect is saturated
scaddenp @768
... - if you look at Trenberth diagram you will notice incoming energy flux at earth surface is 161+333 W/m2 (of course balanced by the same outflow), whereas incoming energy flux at TOA is only 341W/m2. These of course are measured values. How does your unconvential view of physics account for this?
I do not address energy balance issues other than those within the atmosphere which apply to the CO2 band saturation issue. I assume a given (but adjustable) intensity value for the 15 micron absorption band at the surface, and see how this radiation is attenuated with respect to altitude. By tuning this intensity value and possibly the absorption factor, we should (hopefully) be able to obtain a balanced energy solution that is also radiometrically correct. From what I have found so far, however, I believe it would be quite at "stretch" to avoid band saturation since we CO2 is such a strong absorber at 15 microns.
-
CallItAsItIs at 20:33 PM on 27 November 2024CO2 effect is saturated
Eclectic @767
Why must conservation of energy "hold for each frequency independently of the others" ?
Because when we break down the EMR into the sum of contributions from the different frequencies it contain, we find that each such contribution is incoherent relative to the others. This means that the energy flux of the entire distribution is simply the sum of the energy fluxes from each contribution. Now, if this is unclear to you, please understand that I cannot pack an entire radiometry textbook into this comment space.
And your Demonic suggestion that seems to imply that atmospheric molecules cannot (in bulk) gain energy from neighbouring molecules . . . is another novel idea that requires your explanation.
No, that idea is not so novel since the occurrence you describe would violate the second law of thermodynamics.
And all this time, you have avoided the IR role (and other roles) of H2O molecules.
That's right since I am only addressing the issue of CO2 band saturation and not H2O greenhouse warming which is completely different.
-
scaddenp at 18:44 PM on 27 November 2024CO2 effect is saturated
CallItAsItIs - if you look at Trenberth diagram you will notice incoming energy flux at earth surface is 161+333 W/m2 (of course balanced by the same outflow), whereas incoming energy flux at TOA is only 341W/m2. These of course are measured values. How does your unconvential view of physics account for this?
-
Eclectic at 18:21 PM on 27 November 2024CO2 effect is saturated
CallItAsItIs @765 ; @766 :-
Each of your "explanations" generates the need for more explanations.
e.g. ~ Why must conservation of energy "hold for each frequency independently of the others" ? [unquote]
[ Even the famous scientific pioneers of the 19th century would be scratching their heads over this novel idea of yours. ]
And your Demonic suggestion that seems to imply that atmospheric molecules cannot (in bulk) gain energy from neighbouring molecules . . . is another novel idea that requires your explanation.
And all this time, you have avoided the IR role (and other roles) of H2O molecules.
And you have not explained why your idea of "TOA" is so very different from that of the mainstream atmospheric physicists.
Yessir, there's much for you to explain ~ to readers and to yourself.
-
CallItAsItIs at 16:54 PM on 27 November 2024CO2 effect is saturated
Bob Loblaw @762
The catch is that all CO2 molecules are continually emitting radiation. This represents an energy loss. And they make up for that energy loss by colliding with other molecules (such as N2 and O2) and gaining energy when those molecules have higher energy.
And how do they get this extra energy? — from Maxwell's Demons! (LOL)
-
CallItAsItIs at 16:37 PM on 27 November 2024CO2 effect is saturated
Bob Loblaw @764
Come on, Bob! Learn some physics!
What we call sources of energy depends on our system and what we are trying to determine. In the case of the greenhouse effect, we are trying to determine the warming of the atmosphere due to GHGs tapping energy from the terrestrial IR radiation rising from the surface. This means that the upwelling terrestrial IR radiation is the source. The GHGs catch energy in the form of photons from this source, and convert it to kinetic energy of the atmospheric gases (including the GHGs). These GHGs, however, are not sources since they contribute no energy of their own.
The sun also is a source of energy since it puts out IR radiation which is absorbed by the GHGs and converted into thermal energy in the same manner as the terrestrial IR radiation. The atmosphere's primary source of thermal energy, however, is the upwelling terrestrial radiation since IR radiation is more at the "tail-end" of the solar spectrum.
If we are interested in determining the temperature of the "solid" subterranean earth, then the sun becomes our primary source. In this case, the earth absorbs the EMR from the sun which is mostly in the visible spectrum since that is where the peak solar emissions occur. Also, the atmosphere is transparent to visible EMR (ie. light). The down-welling terrestrail radiation from the atmosphere is another a source, but a much weaker one.
Now that we have (hopefully) gotten it straight as to what is meant by sources of energy, let's get back to the problem-at-hand of assessing saturation of the 15 micron absorption band of CO2. In this case, our source of energy is the upwelling terrestrial radiation within this absorption band. Since the contributions to the total upwelling EMR at different frequencies involve different photons, conservation of energy must hold for each frequency independently of the others. This enables us to use the Beer Lambert law to evaluate the attenuation of each frequency component of the upward-bound IR. And, as indicated in previous posts, intensity contributions within the 15 micron band become pretty miniscule at altitudes well below the TOA.
-
Bob Loblaw at 11:22 AM on 27 November 2024CO2 effect is saturated
CallItAsItIs:
Since you are really big on making sure we agree on basics and terminology, could you please provide us with your definition of "sources of energy"?
Try to remember the principle of conservation of energy while you are at it.
-
Bob Loblaw at 11:19 AM on 27 November 2024CO2 effect is saturated
CallItAsItIs @ 761:
You say:
Convection and conduction are mechanisms for transferring energy. They are not sources of energy.
And that is why the Trenberth diagram uses the title "Global Energy Flows".
It may be news to you, but radiation is also a mechanism for transferring energy. Have you never noticed that radiation travels from one place to another? It does so fairly quickly - as a rough approximation, at the speed of light.
-
Bob Loblaw at 11:14 AM on 27 November 2024CO2 effect is saturated
CallitAiItIs @ 760:
Once again, you fail to look at the entire system, and fail to understand what is being said to you.
What Charlie Brown says in 756 is correct. Indeed, his statement that "CO2 molecules collide with N2 and O2 to come to thermal equilibrium (i.e., same temperature)" means exactly the same thing as your statement "CO2 molecule releases the energy it gained from the photon absorption which then becomes kinetic energy of both molecules, thereby raising temperature. "
The catch is that all CO2 molecules are continually emitting radiation. This represents an energy loss. And they make up for that energy loss by colliding with other molecules (such as N2 and O2) and gaining energy when those molecules have higher energy.
The energy transfer via collision between CO2 and other molecules works in both directions, depending on the relative energy level of the individual molecules. (You can't talk about temperature of an individual molecule, as "temperature" is a bulk property of many molecules. Average kinetic energy.)
I have previously pointed you to this blog post at Eli Rabett's, where this is explained well.
Please read some of these additional links you have been pointed to. You continue to embarrass yourself by making elementary errors in physics. Until you unlearn your misconceptions, it is very difficult for you to get an accurate understanding of this subject.
-
CallItAsItIs at 11:07 AM on 27 November 2024CO2 effect is saturated
Bob Loblaw @754
Convection and conduction are mechanisms for transferring energy. They are not sources of energy.
-
CallItAsItIs at 10:58 AM on 27 November 2024CO2 effect is saturated
Charlie_Brown @756
It works like this: photon is absorbed by CO2. CO2 molecules collide with N2 and O2 to come to thermal equilibrium (i.e., same temperature). CO2 molecule emits photon. The net effect at equilibrium is a pass-through of energy unless there is a change that upsets equilibrium.
Wrong! It works like this: Photon is absorbed by CO2. Energized CO2 molecule collides with N2 or O2. In this collision, CO2 molecule releases the energy it gained from the photon absorption which then becomes kinetic energy of both molecules, thereby raising temperature. At the same time, of course, the CO2 molecule returns to it original state, ready to absorb another photon.
In your explanation, warming of any of the atmospheric gases would not be possible with violating energy conservation.
-
Bob Loblaw at 08:16 AM on 27 November 2024CO2 effect is saturated
Oh, and when CO2 (or anything else) "absorbs a photon", it does not store the photon, it just stores the energy. The photon ceases to be. It is no more. It is a "late" photon.
When CO2 (or anything else) emits a photon, it does not get one out of storage. It creates one using energy stored elsewhere in the molecule.
Energy is conserved, but there is no such law as "conservation of photons", as Charlie Brown has already stated in comment 757.
-
Bob Loblaw at 08:10 AM on 27 November 2024CO2 effect is saturated
Charlie Brown @ 757:
Minor correction - yes, individual photons have the properties of both wavelength and frequency. Part of the weirdness of the particle/wave duality of light/EM radiation.
The wavelength, frequency, and energy of a photon are all tied together. If it is a 15um photon, that also sets its frequency and energy level. All 15um photons are the same.
A "stream" of photons is not like a stream of water where everything is connected. Even a stream of photons is just a bunch of individual photons passing one at a time past a point in space. When silicon diodes are used as radiation sensors, they are basically "photon counters".
When a higher temperature means more energy emitted at a specific wavelength, it just means "more photons", not "more energy in each photon".
-
Charlie_Brown at 04:27 AM on 27 November 2024CO2 effect is saturated
Clarification: Pass-through of photons is more descriptive than pass-through of energy. Photons don't have wavelength so it risks mixing up the concept of electromagnetic radiation as waves or particles. However, they are emitted at a specific frequency so a stream of photons can be described as wavelength. A column of the atmosphere has conservation of energy, not conservation of photons. Radiant energy follows the temperature profile of the atmosphere. Stefan-Boltzmann Law: Intensity = emittance (specific to wavelength) x Stefan Boltzmann constant x absolute temperature to the 4th power.
-
Charlie_Brown at 03:04 AM on 27 November 2024CO2 effect is saturated
CallItAsItIs @748,
Since you ask a specific question: Kirchhoff’s Law is absorptance = emittance (at thermal equilibrium). It works like this: photon is absorbed by CO2. CO2 molecules collide with N2 and O2 to come to thermal equilibrium (i.e., same temperature). CO2 molecule emits photon. The net effect at equilibrium is a pass-through of energy unless there is a change that upsets equilibrium.
You emphasize that the 15-micron absorption band (by which I think you mean the approximately 14-16 micron band and not a few 14.9 micron peak lines) absorbs completely within 10 meters of the surface. @725 you say “CO2 can still emit IR radiation (at any wavelength) but can no longer absorb within this band.” @751 the 15 micron absorption band of CO2 is strong enough to pack the thermal radiation from the entire band into a layer at the surface just a few tens of meters thick.” On the other hand, @740 you seem to acknowledge that the spectra showing upward IR within the band are correct, not just at 70 km but also at lower altitudes. If you were consistent, there would be zero intensity for the band because it would have all been absorbed at low altitude. Maybe I give you too much credit there, since your interpretation of the spectra was not correct and you said CO2 can still emit but no longer absorb, so maybe you were not being inconsistent but just do not apply Kirchhoff's Law.
-
Bob Loblaw at 00:09 AM on 27 November 2024CO2 effect is saturated
As a followup to my comment at 753, I grabbed the Trenberth diagram from this SkS post, where it is figure 6. The caption for that figure gives the source as this.
-
Bob Loblaw at 23:43 PM on 26 November 2024CO2 effect is saturated
...and to respond to CallItAsItIs @ 751, where he says:
The primary mechanism for the sun warming the earth is the absorption of visible light (from the sun) into the solid portion of the earth which then acts as a near blackbody. This blackbody radiator then warms the atmosphere by conduction and convection.
This, and the rest of your comment at 751, is essentially correct. Yet for some strange reason, you completely ignore all this atmospheric heating by conduction and convection when you claim that there is no possible source of energy to drive emission of 15um radiation within the atmosphere. In comment 741 (responding to my comment 732, where I said the atmosphere is a source of IR radiation), you stated:
Are you saying that the atmosphere heats itself!? Wrong! This would violate energy conservation. In order to to heat the atmosphere, we must bring in IR from outside the atmosphere. In the case of greenhouse warming, this is the 288 K IR eminating from the surface of the earth.
So, which is it? Does conduction and convection add energy to the atmosphere? Energy that is then available to be emitted as IR radiation? Or is it solely the input of IR radiation that can provide a source of energy for the emission of IR radiation?
The inconsistency of your arguments is astounding.
-
Bob Loblaw at 23:28 PM on 26 November 2024CO2 effect is saturated
CallItAsItIs @ 749:
Yes, we need to get some basics straight. You say:
In this problem, we are trying to assess warming of the atmosphere due to IR radiation eminating from the surface of the earth. This earth-emitting IR is estimated as a blackbody at about 288 deg. K, although we do consider it to be adjustable.
For energy conservation, we must take this to be the only source of energy causing addtional warming to the entire atmosphere.
...and at this point, you have the basics horribly, horribly wrong. Energy conservation applies to all forms of energy. There is no "energy conservation" that applies solely to IR radiation. Energy is conserved in a system that obtains all energy as solar radiation, and emits the same amount of energy solely as IR radiation. When you isolate one form or another, there is absolutely no requirement that solar energy be conserved, or IR energy be conserved.
From this basic misunderstanding on your part, you have created a cartoon physics that bears no resemblance to reality.
You then continue with:
The term thermal radiation is used to denote the distribution of EMR when thermal equilbrium is reached.
This, frankly, is "not even wrong". Try reading Wikipedia's page on thermal radiation. The opening paragraph starts with:
Thermal radiation is electromagnetic radiation emitted by the thermal motion of particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation.
Since you can't even get the basics right, the rest of your argument is completely illogical.
As for Eclectic's reference to the Trenberth energy diagram, here it is. Note that the only "conservation of energy" rules that applies is to the entire diagram as a whole - not the individual components (IR, solar, etc.)

-
Eclectic at 19:54 PM on 26 November 2024CO2 effect is saturated
CallItAsItIs @751 & prior :-
Yes indeed, it is "ultimately the sun that drives warming of the entire earth, both the solid part and the atmosphere".
And for practical purposes, we can ignore the (fission) heat generated subterraneously, and heat generated by tidal motion, and heat generated by combustion of fossil fuels etcetera.
But when discussing atmospheric physics, it would be substantially wrong to ignore the direct heating of the atmosphere by sunlight (as short-wave radiation, including UV radiation). There is a famous "cartoon" by Dr Trenberth, giving a summary of the various energy flows in the terrestrial atmosphere ~ quite colorful, though the exact figures utilized are perhaps only accurate to about +/- 1%.
Forgive me, but I feel you are pulling my leg, when you say (with your atmospheric interest) that you have not heard of Trenberth. Just like I would feel when a Rocket Scientist says he has not heard of Newton.
-
CallItAsItIs at 19:24 PM on 26 November 2024CO2 effect is saturated
Eclectic @750
Well, it is ultimately the sun that drives warming of the entire earth, both the solid part and the atmosphere. The primary mechanism for the sun warming the earth is the absorption of visible light (from the sun) into the solid portion of the earth which then acts as a near blackbody. This blackbody radiator then warms the atmosphere by conduction and convection.
It should be noted that convection is important for the CO2 greenhouse effect to work since the 15 micron absorption band of CO2 is strong enough to pack the thermal radiation from the entire band into a layer at the surface just a few tens of meters thick. Without convection, this would give us a very hot surface. Also, however, there would be a steep temperature gradient near the surface which would result in a strong pressure gradient. This pressure gradient would then drive updrafts that would carry excess heat away from the surface. In this manner, convection stops excessive heating of the bottom layer of the atmosphere.
Finally, I am not familiar with Trenberth's summary. Could you give me a link or a reference?
-
Eclectic at 18:08 PM on 26 November 2024CO2 effect is saturated
CallItAsItIs @749 :-
Please do not forget about the Sun warming the atmosphere.
Also, convection and H2O phase changes, etc.
I'm fairly sure you were going to mention all these other factors, and also bring Trenberth's summary into the discussion. After all, we would be remiss if we ignored the Big Picture and focused entirely on only IR photons and the abstract "Laws of Physics".
-
CallItAsItIs at 16:16 PM on 26 November 2024CO2 effect is saturated
Bob Loblaw @ 744
From this comment and several others of yours, I believe we need to get some basics straight so that you and I are consistent in our use of various term (eg. thermal radiation). Also, we need to be certain we are even working the same problem. Although I am addressing this message specifically to you, Charlie_Brown, Eclectic, and scaddenp are certainly welcome to tune in.
In this problem, we are trying to assess warming of the atmosphere due to IR radiation eminating from the surface of the earth. This earth-emitting IR is estimated as a blackbody at about 288 deg. K, although we do consider it to be adjustable.
For energy conservation, we must take this to be the only source of energy causing addtional warming to the entire atmosphere. This does not mean, however, that energy can't change forms within the atmosphere. Indeed, this is what happens in establishing thermal equilibrium. But there cannot be any change in total energy in the process. The atmosphere does not consume fuel and has no means of generating new energy.
Next, we assume that the molecules and photons are of sufficient number that it makes sense to talk about smoothly varying densities, fluid velocities, temperatures, and pressures. Also, we assume that thermal equilibrium is maintained at least locally. This is what enables us to discuss different temperatures at different altitudes.
The term thermal radiation is used to denote the distribution of EMR when thermal equilbrium is reached. If the atmosphere was totally absorptive at all EMR frequencies, then the thermal radiation would simply be given by the Planck distribution. Of course this is not the case for the entire EMR spectrum, but at wavelengths near 15 microns or other bands that are strongly absorptive, the atmospheric radiance at those wavelengths comes close to matching those of a blackbody at the temperature where the measurement is taken.
Applying this concept of thermal radiation to the TOA (about 70km altitude), we find that CO2 in effect forms a narrow bandwidth "blackbody" at the 15 micron wavelength. At this point, the detectors pick up on the emissions from this "blackbody" since there is no more CO2 at the TOA to attenuate them. This is why the last three curves that Bob Loblaw posted in 731 all show the same intensity (about 6 W/m^2) and the same temperature (about 220 deg. K) of the 15 micron band. Emissions from this band at lower altitudes and higher temperatures have all been absorbed.
At this point, we still need to discuss absorption coefficients and the Beer Lambert equation which govern the conversion of upwelling IR radiation from the surface to thermal energy of the atmosphere, but I think I will wait for feedback before I spend any more time at this.
-
CallItAsItIs at 13:27 PM on 26 November 2024CO2 effect is saturated
Charlie_Brown @745
Could you please explain to me how my postings are not consistent with Kirchhoff's Law?
-
Bob Loblaw at 03:54 AM on 26 November 2024CO2 effect is saturated
Regarding Charlie Brown's comment at 745 (and earlier) about thinking of Beer's Law from the perspective of the top of the atmosphere:
- Beer's Law is a probability relationship. It tells us the likelihood that a certain number of photons will be absorbed over a given distance (which is better described as a given number of absorbing molecules - it's the mass of absorbing molecules that matters).
- An individual photon maybe absorbed at the start of that path, in the middle, or at the end - or not at all.
- Beer's Law just says "on average, this portion will be absorbed".
- As we move away from the source, the probability that a photon will get that far decreases.
- When looking at what comes out of the top of atmosphere, Beer's Law tells us the probability that a particular photon came from a source at distance X (again, "distance" = number of molecules).
- There is a higher probability that it came from a close source than a distant source.
As we follow the 15um flux up from the surface, Beer's Law tells us that at each height, we're probably looking at more photons that were emitted at heights slightly below where we are, and fewer photons that were emitted far, far below where we are. There will always be 15um photons flowing upward, though.
-
Bob Loblaw at 03:39 AM on 26 November 2024CO2 effect is saturated
I feel as if I'm on the verge of beating a dead horse - no idea if CallItAsItIs will respond to the latest comments, but....
I think it is useful to provide a graphic published in a 1967 paper by Manabe and Wetherald.
Figure 16 looks like this:
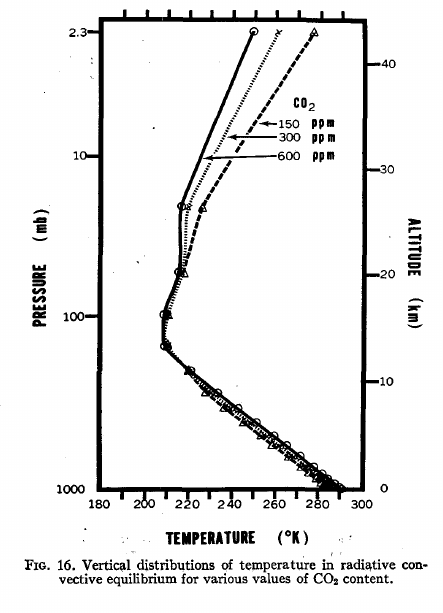
Note that increasing CO2 increases surface temperatures, but leads to cooling in the stratosphere. Why? Many factors, but one of them is that by adding CO2, the overall emissivity of the atmosphere increased - so the atmosphere can emit the same IR radiation to space at a lower temperature. Kirchoff's Law at work - both absorptivity and emissivity change in unison.
But CallItAsItIs has not yet caught up with science from 1967, so he believes that only the emission of "thermal radiation" is being picked up by satellite sensors, and there is no emission from CO2.
-
Charlie_Brown at 03:13 AM on 26 November 2024CO2 effect is saturated
Bob’s points are spot on. CallItAsItIs sometimes uses Kirchhoff’s Law and sometimes forgets about it. There is one more interesting point that I would like to add about interpreting the graphs. They demonstrate conclusively that there are sufficient CO2 molecules to form an emitting layer (emittance = 1.0) at the bottom of the stratosphere. This shows the value of interpreting Beer’s Law by looking down from the top of the atmosphere.
CallItAsItIs misses the interpretation when he says “the intensity at 15 microns approaches a value corresponding to a 220 K blackbody. This, in turn, corresponds to an altitude of about 70 km, above which there isn't much of an atmosphere.” 220 K also corresponds to an altitude of 24 km and that is the source of radiant energy loss to space in the 14-16 micron band. How do I know? Look at the 15-micron peak, which is composed of a few very strong emittance lines, in the spectrum at 50 km. The temperature at that altitude is 270 K, but the top of the peak corresponds to the Planck distribution of 241 K. By Beer's Law, there are not enough CO2 molecules to bring the emittance to 1.0 for the strongest peak.
-
Bob Loblaw at 03:02 AM on 26 November 2024Fact brief - Does manmade CO2 have any detectable fingerprint?
Rkcannon:
The paper in question, by Skrable et al, was discussed shortly after it appeared, over at AndThenTheresPhysics.
Note that the ATTP blog mentions a total of five rebuttal comments that were submitted to the journal.
Executive summary: the paper is a piece of junk.
-
Eclectic at 02:33 AM on 26 November 2024Fact brief - Does manmade CO2 have any detectable fingerprint?
Rkcannon @1 :-
you mention a 2022 paper published in "Health Physics" by authors Skrable, Chabot, et al.
I gather that the journal "Health Physics" is primarily concerned with the topic of radioactivity effects on human health.
Presumably the Skrable paper had been rejected by reputable journals that normally published general scientific matters (including the physics of climate science).
Judging by Skrable's Abstract, the authors have made a colossal fundamental error in their understanding of the carbon cycle (involving the movement of CO2 into and out of Earth's atmosphere). And hence their conclusion is grossly erroneous.
The puzzle is : how did the journal Editor fail to see this error.
-
rkcannon at 01:29 AM on 26 November 2024Fact brief - Does manmade CO2 have any detectable fingerprint?
Please comment on the following paper "World Atmospheric CO2, Its 14C Specific Activity, Non-fossil Component, Anthropogenic Fossil Component, and Emissions (1750–2018)" concluding "We determined that in 2018, atmospheric anthropogenic fossil CO2 represented 23% of the total emissions since 1750 with the remaining 77% in the exchange reservoirs. Our results show that the percentage of the total CO2 due to the use of fossil fuels from 1750 to 2018 increased from 0% in 1750 to 12% in 2018, much too low to be the cause of global warming." Fact or fiction?
-
Bob Loblaw at 00:55 AM on 26 November 2024CO2 effect is saturated
To try to make a few points, independent of CallItAsItIs's confusion:
- All objects with a temperature >0 Kelvin emit radiation. (Planck's Law)
- As temperature increases, this shifts to shorter wavelengths (higher frequency).
- When an object primarily emits in the IR range, this is often called "thermal radiation", but radiation consists of photons, regardless of wavelength.
- At a specific wavelength (e.g. 15um), all photons are the same. They have no memory of what emitted them, and no memory of what temperature that object was at.
- There are no "thermal radiation" 15um photons that act differently.
- There are no "288K" 15um photons that act differently.
- There are no "surface-emitted" 15um photons that act differently.
- There are no "CO2 can't absorb this" 15um photons that act differently.
- There are no "CO2 will absorb this" 15um photons that act differently.
- They are just "15um photons".
- Gases are highly selective with respect to what wavelength of photons they will emit. This has to do with energy levels in the gas molecule: the structure only allows for certain energy levels, and as the molecule drops from one level to another, a photon is emitted with that energy - and photon energy is directly related to its wavelength.
- Gases will absorb photons at the same wavelengths they emit. (Kirchoff'sLaw)
- This does not mean that energy obtained from absorbing a 15um photon has to be emitted as a 15um photon.
- Energy lost by emitting a photon has to match the energy in that photon, but the energy can come from anywhere. Absorption of radiation at other wavelengths, obtaining energy via collision with other molecules (the dominant factor in the atmosphere), chemical reactions, etc.
To call it as it is, it appears that CallItAsItIs fails to understand many of these essential aspects of physics and radiation transfer.
-
Bob Loblaw at 00:25 AM on 26 November 2024CO2 effect is saturated
CallItAsItIs @ 740:
Are you actually paying attention to what you are saying?
- You have been claiming that there is no 15um IR radiation above your "extinction height" (10m) for CO2 to absorb.
- I showed graphs (albeit modelled) with non-zero 15um radiation at altitudes from 10km to 70 km.
- Now, you claim that the presence of that 15um IR radiation from 10km through 70km "tend[s] to show that I [you] am right", because "the detectors are only picking up some thermal radiation from the TOA".
The level of physics denial is astounding. For your explanation to be correct, the entire atmosphere between the lower near-surface layers and the top of atmosphere layers cannot emit IR radiation in the 15um band. In your version of "physics":
- There is lots of 15um IR radiation emitted upwards at the surface...
- ...but a short distance above the surface, suddenly there is no upward emission of 15um radiation...
- ...and there continues to be no upward emission of 15um radiation as we move higher and higher in the atmosphere....
- ...until suddenly, at the top of the atmosphere, we suddenly find conditions that allow for the emission of 15um radiation so that the satellite sensors can pick it up.
- ...and somehow "thermal radiation" is some different entity that does not exist in all those layers between the surface and the TOA. It only appears at the TOA, where you need it to "explain" what is measured from space.
You are creating a physics-free zone between the surface and the TOA. In spite of the fact that the entire atmosphere has a temperature above 0K, and therefore contains thermal energy, and therefore emits thermal radiation (including 15um radiation, because the CO2 that is present can do that...), you have declared that none of that physics applies and none of the upwelling 15um radiation that is measured between the surface and the TOA exists.
...and then in comment 741, you wander off into a tangent claiming that my explanation in comment 732 requires that the atmosphere "heats itself" and "violate[s] energy conservation". That is more physics denial. Any object with a temperature >0K contains thermal energy. And the laws of physics state that any object >0K will emit radiation.
When a molecule emits radiation, it loses energy (conservation of energy maintained) and will cool, but each molecule is capable of regaining energy by collisions with other molecules, or by absorbing radiation (in any wavelength - the absorption and emission do not need to be in the same wavelength).
Remember in comment 722 when you said?:
In the case of CO2 and the 15 micron absorption band, the N2 and O2 molecules in the surrounding air collide with energized CO2 molecules which causes the extra energy (from absorbed photons) to be converted into thermal energy, thereby raising the air temperature.
The opposite is also true. CO2 can gain energy from N2 and O2 through collisions, when the N2 and O2 molecules are at higher thermal energy levels than the CO2. That is what predominantly drives the "thermal radiation" emitted by CO2 at all levels in the atmosphere. You can read the technical details in this post at Eli Rabett's blog.
-
Eclectic at 17:36 PM on 25 November 2024CO2 effect is saturated
Charlie_Brown @739 :-
Our two-way conversation is getting rather off-topic for this thread.
I am sure that we two are "on the same side" regarding AGW/science. But there is clearly a large semantic discrepancy with our individual understandings of the meaning of certain English words ~ and it would take far too long for us to negotiate a mutual agreement on the semantics.
Let it slide.
The point which I found interesting (and which I always find interesting in reading all the nonsenses, bad science, and Motivated Reasonings to be found on the WUWT website) . . . is that our new friend [viewed @722 ; @723 ; @726 ; @730 ; @740 ; and @741 ] and suchlike people ~ are coming to pseudo-science conclusions because of poor logic and poor understanding of the physical universe of particles & photons. They are hampered by their confusions of realities vs abstractions (e.g. the mental constructions achieved by the great scientists of the 18th, 19th, and later centuries. Constructions which have been dignified by the label "Laws" ) .
[ Off-hand, I do not know of a suitable thread on SkS , for the discussion of the philosophy of science as expressed through human psychology & semantics. ]































 Arguments
Arguments



























