The human fingerprint in the daily cycle
Posted on 20 November 2010 by John Cook
During the day, the sun warms the Earth's surface. At nighttime, the surface cools by radiating its heat out to space. Greenhouse gases slow down this cooling process. This is why deserts cool so much at night. Water vapour is a strong greenhouse gas and the dry desert air traps much less heat than more humid areas.
A more extreme example is the moon which has no atmosphere. At nighttime, there are no greenhouse gases to trap the outgoing heat. Consequently, the difference between day and night is more extreme with daytime temperatures getting up to around 118°C and nighttime temperatures falling below -168°C. In other words, the stronger the greenhouse effect, the smaller the difference between daytime and nighttime temperatures.
We are currently experiencing global warming. If an increased greenhouse effect is a significant part of this warming, we would expect to see nights warming faster than days. There have been a number of studies into this effect, which confirm that this is indeed the case. One study looked at extreme temperatures in night and day. They observed the number of cold nights was decreasing faster than the number of cold days. Similarly, the number of warm nights was increasing faster than the increase in warm days (Alexander 2006).
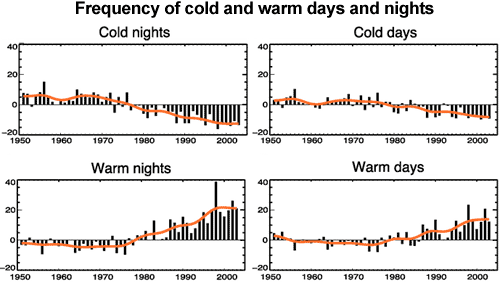
Figure 1: Observed trends (days per decade) for 1951 to 2003 in the number of extreme cold and warm days and nights per year. Cold is defined as the bottom 10%. Warm is defined as the top 10%. Orange lines show decadal trend (IPCC AR4 FAQ 3.3 adapted from Alexander 2006).
The difference between daytime and nighttime temperatures is also known as the diurnal temperature range (DTR – the difference between minimum and maximum daily temperature). An increased greenhouse effect should cause the DTR to decrease. Over the last 50 years, DTR over land has shown a large negative trend of ~0.4°C (Braganza et al. 2004). The reason for the falling DTR is because nighttimes have been rising faster than daytime.
The daily cycle also offers interesting insights into climate change over the 20th Century. From the 1950s to early 1980s, global temperatures cooled slightly. A large contributor to the cooling was "global dimming" from 1958 to 1990 where less sunlight made it to the Earth's surface due to air pollution. However, over this period, the nighttime minimum temperature increased. While global dimming was cooling daytime temperatures, the increased greenhouse effect was warming the nights (Wild et al 2007). Even during mid-20th Century cooling, greenhouse warming was percolating away while we were sleeping.































 Arguments
Arguments






























@Ddahl44 @149
No, that is not correct. Just looking at the static concentrations does not give you the answer you want. It is the change in conditions that you should be looking at. CO2 concentration has increased so that has led to a rise in the temperature. In turn that has led to an increase in the amount of water vapour in the atmosphere, which results in even more of a rise in the temperature. So there is a feedback effect.
For details on contribution of the gases to the overall GHE which keeps us from freezing, see this paper here. I dont see how cloud seeding would help - the amount of water in the atmosphere is dependent on temperature (Clausius-Clapyron relation) hence water vapour acts as a feedback to any other variable that is changing the temperature.
JohnSeers - my nonclimate scientific mind needs to understand stasis before I can understand change. Eclectic states each molecule (H2O or CO2) can absorb a photon. If this is correct, then at any point in time, assuming H2O makes up 2% of the molecules in the atmosphere, are not H2O molecules absorbing 70x the photons of CO2? How does CO2 jump from 1.5% of the work to 20%, regardless if it is doubled or not? I need to understand this before I work on feedback loops and changing the variables. Thanks.
Ddahl44 @153 ,
If I may make a brief and rather over-simplified reply :-
H2O and CO2 operate at different "transmission bands" of InfraRed radiation ~ so they are not in competition, and so can't be directly compared.
A second aspect, is that (effectively) the IR loss (to outer space) is occurring from molecules at very high altitudes in the atmosphere, where the temperatures are so cold that very little H2O is present in vapor form ~ unlike the case of CO2 (which does not condense at these temperatures, and so maintains its relative concentration of 0.04% ).
Ddahl44 - the Schmitdt et al paper that I pointed you to in 152 has the detail for current atmosphere. Did you look at it? (short answer - the calculation is a lot more complicated than you think. You cannot treat the atmosphere as a single layer, nor are the responses to IR for each type of molecule the same).
The really gruesome detail is encompassed in the Radiative Transfer Equations. A lot of teaching resources around the net for these. eg here and here.
Ddah144,
Your initial comment on this thread @142 made quite an issue of "the moon’s huge day to night temperature swings" which doesn't seem to have been addressed properly. You correctly point out that the massive size of the change in lunar day-to-night temperature is due to the month-long Lunar day. The graph below shows the equitorial lunar temperature and the temperature range remains high all the way from the equator almost to the poles - even at 75º of latitude it has only dropped from a 300K swing to 200K.
The portion of this lunar graphic of interest when considering the equivalent effect for a 24 Earth-hour rotation would be the 0.8 Lunar-hours centred on the Lunar average temperature. That would suggest a day-to-night equatorial temperature range of something like 80ºC. A more accurate calculation (the graphic below provided by climate skeptic Roy Spencer) shows an equitorial range of about 70ºC, a lot lower than the actual range for a planet with a GHG atmosphere. For instance Singapore has (or more correctly 'had') an average daily maximim of 30.3ºC and daily minimum of 23.5ºC, thus a range of just 7.2ºC.
@Ddahl44 @153
The significant point about "stasis", that is no change, is that there will not be a temperature rise. The situation is static.
In a changing system, if you add a molecule of water vapour to the atmosphere it will be rained out very quickly in a matter of hours/days and the system will still be at the same temperature. The carrying capacity of the atmosphere will not have changed.
If you add a molecule of carbon dioxide it will stay in the atmosphere for a long, long time (100000 years?). In that time it will capture (and release) a photon many times and add a small amount of heat to the system. It will slow the escape of heat to space. The temperature will not return to equilibrium like the water vapour molecule. In addition the carrying capacity of the atmosphere for water vapour will have increased which leads to a feedback rise in temperature.
MA - thanks for the graphs. Lord(!) Kelvin temperatures I haven’t seen in years. We, in medicine, have trouble with converting C and F back and forth. It looks by the graph that the huge temperature swings on the moon are not so much due to the long lunar nights, but rather the long lunar days. Once it gets dark the temperature drops by 30-35 degrees C over 14 earth days. To extrapolate then, during a 12 hour earth night the temperature drops a little over 1 degree C. Not much. However from dawn to midday over 7 earth days the temperature rises an incredible 300 degrees. Extrapolation is more difficult for day changes since we’re on a curve and at least in Kansas, temperatures peak later in the day. But 6 hours of Earth day heating would increase the moon temperature by about 11 degrees. This is still more of a temperature change than Singapore, but to be fair to the moon, actually less of a change than a more comparable arid Earth locales, like say Phoenix, Cairo or Baghdad. They each had day/night differences yesterday of between 25 and 30 degrees F.
scaddenp,
I read the article, thanks. From what I understand of it, eclectic, water and CO2 are in competition because they share some wavelengths of absorption. What I find troubling is Schmidt splits the effect of CO2 and H20 for their shared wavelengths 50/50. How can this be if there are 70x as many water molecules as CO2? I realize, too, that H20 vapor does not hang out in the upper levels of the atmosphere. But surely the most important greenhouse molecules must be those closer to the earth’s surface, where weather is created and the heat is felt. The “hot” CO2 molecules ahigh are less abundant at lower pressures and have less to bounce into, right? JohnSeers, I realize there is a water cycle just as there is a CO2 cycle. But at any one time, despite evaporation, rain, and ocean CO2 absorption, there are still 70x as many water molecules as CO2. Before we leave the moon, I just saw a Business Insider article that linked higher temperatures on the moon with astronauts stirring up dust on their brief visits. So man is causing lunar warming?! (would link it but couldn’t). Thanks all.
" But surely the most important greenhouse molecules must be those closer to the earth’s surface, where weather is created and the heat is felt."
GHG at all levels, capture radiation and cause re-radiation downward. Upper level GHG are very important.
"Schmidt splits the effect of CO2 and H20 for their shared wavelengths 50/50." No he doesnt. See table 1.
From Schmidt:
[17] If the absorbers are grouped in a simple manner, i.e., water vapor, clouds, CO2 and all other factors, and some simplifying assumptions made, it is relatively straightfor- ward to infer the overlaps and estimate the net attribution of the total greenhouse effect to the individual constituents. Following KT97, given an overlap between two absorbers, an obvious allocation is to split the difference, i.e., if 5% of the net LW radiation could be absorbed either by water vapor or CO2, then each is allocated 2.5%.
But following KT97, that is splitting the overlap at the probability level. Concentrations still matter.
Part of my residency training in Emergency Medicine and continuing education involves review of scientific articles of relevance to our specialty including controlled prospective trials, literature reviews, and policy statements. Schmidt’s language, specifically his allocations based on estimated attributions and inferences is quite foreign at least to scientific review in my field. At a minimum it seems somewhat arbitrary, lacks objectivity, allows for author subjective interpretation of data and opens up the potential of bias. This concerns me if this is a go-to reference in the field.
Perhaps you need to broaden and deepen your knowledge of the terminology used in this field, given that it is far outside your area of expertise.
For example, per Lacis et al 2010:
“Ample physical evidence shows that carbon dioxide (CO2) is the single most important climate-relevant greenhouse gas in Earth's atmosphere. This is because CO2, like ozone, N2O, CH4, and chlorofluorocarbons, does not condense and precipitate from the atmosphere at current climate temperatures, whereas water vapor can, and does.
Non-condensing greenhouse gases, which account for 25% of the total terrestrial greenhouse effect, thus serve to provide the stable temperature structure that sustains the current levels of atmospheric water vapor and clouds via feedback processes that account for the remaining 75% of the greenhouse effect.
Without the radiative forcing supplied by CO2 and the other non-condensing greenhouse gases, the terrestrial greenhouse would collapse, plunging the global climate into an icebound Earth state.”
https://www.nasa.gov/topics/earth/features/co2-temperature.html
https://www.giss.nasa.gov/research/briefs/lacis_01/
https://www.giss.nasa.gov/research/briefs/schmidt_05/
https://www.nasa.gov/topics/earth/features/vapor_warming.html
Schmidt is merely building on a long line of other papers and the paper is effectively a "quick a dirty" for the purposes of informing public discussion. From abstract:
"Much of the interest in these values is however due to an implicit
assumption that these contributions are directly relevant for the question of climate sensitivity."
ie. it doesnt have a lot of relevance to the practise of climate science. Actual model codes are integrate over all gases and all absorbtion bands simultaneously. They reproduce observations of the radiation spectra with exquisite accuracy. Climate depends on how the system plays as a whole and the individual contributions of the gases in any particular atmospheric composition is of little practical interest.