Ocean acidification: Some Winners, Many Losers
Posted on 10 June 2011 by Rob Painting
Numerous lab experiments have shown that ocean acidification is harmful to marine life. Creatures that build chalk-like shells (or skeletons) fare poorly under conditions which mimic the low ocean pH levels expected later this century. This isn't a universal response however; some starfish, brittle stars and sea urchins, seem relatively unaffected by ocean acidification, so it's likely there will be winners and losers as the world's oceans become less alkaline.
Nature's own laboratory
Despite their usefulness, lab experiments are no substitute for the natural environment, and experiments tend to be of short duration too, so the long-term effects of elevated CO2 on marine communities is largely unknown. Luckily there are a couple of locations in the world which mimic ocean acidification. These locations are not perfect analogues for the future, seawater acidity is not held permanently low because of seasonal wind and current variation, for example, but they do give some insight into how marine life might adapt (or not) to ocean acidification over the long haul.
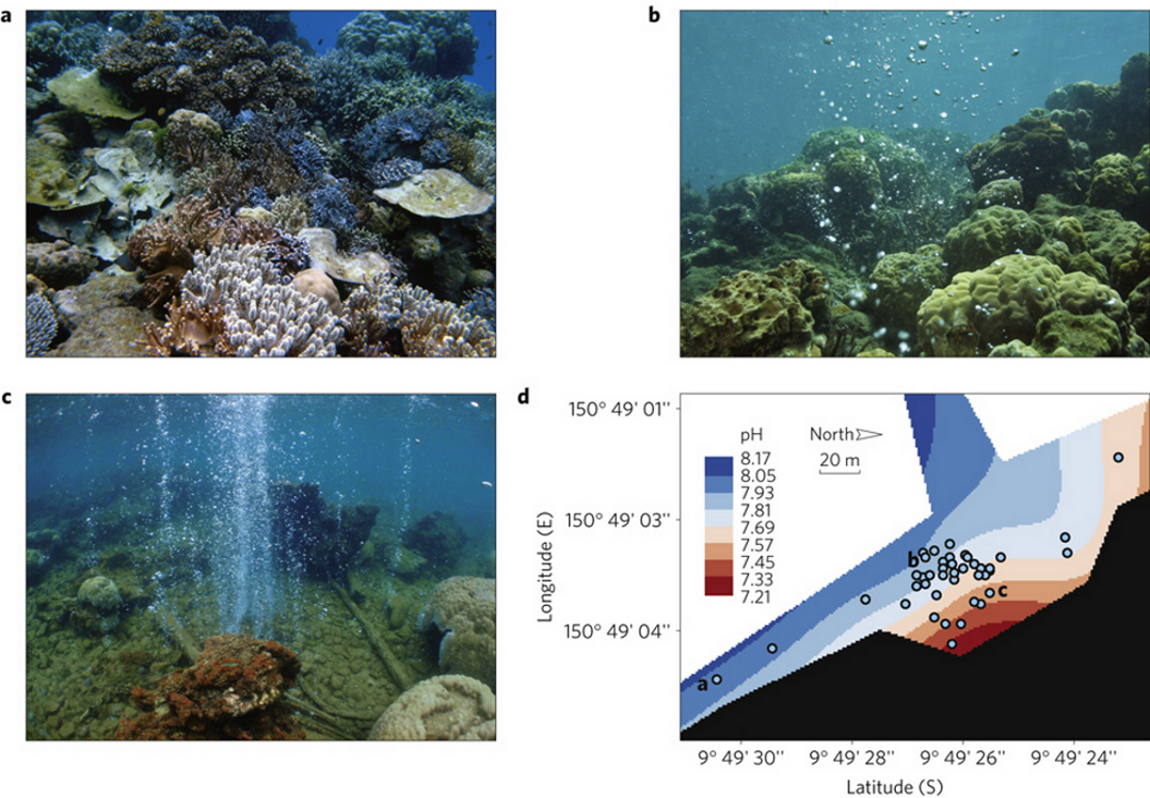
Fabricius 2011 examines the coral reefs in one such area, Milne Bay Province in Papua New Guinea, where cool CO2 bubbles up through natural seeps on the seafloor, thereby lowering seawater pH. The authors examined underwater sites which are equivalent to 3 ocean pH scenarios: a control site, where seawater is at ambient (normal) pH (around 8.1), a low pH site (7.8-8.0), and a very low pH site (below 7.7).
Figure 1 -Seascapes at a, control site (‘low pCO2’: pH~8.1), b, moderate seeps (‘high pCO2’: pH 7.8–8.0), and c, the most intense vents (pH<7.7), showing progressive loss of diversity and structural complexity with increasing pCO2. d, Map of the main seep site along the western shore of Upa-Upasina. Colour contours indicate seawater pH, and the letters indicate the approximate locations of seascapes as shown in a–c.
What the authors found, is probably no surprise: as the level of seawater pH dropped from it's normal value of 8.1, the health of the reef began to deteriorate. Various hard and soft corals, and other hard-shelled marine life, such as cructose coralline algae, disappeared from the seafloor. Only one type of coral was able to tolerate ph as low as 7.8, which is equivalent to 750 ppm of atmospheric CO2: a figure we're on track to reach before the end of the 21st century. Below a pH of 7.7 however, coral reef development stopped dead in it's tracks. The beneficiaries, it turns out, are slime (macroalgae) and seagrasses, with their populations flourishing as seawater becomes more acidic.
Another discovery made by the authors, is that coral reef growth (calcification) was 30% lower than is the norm for coral reefs at similar latitude (distance from the equator), and both the low and ambient pH sites exhibited the same condition. The Milne Bay area has experienced repeated mass coral bleaching events in the last 20 years, so this has probably played a role in the low growth rates. This is consistent with the latest research showing coral growth rates have diminished worldwide in recent decades.
Natural CO2 seeps in the Mediterranean
The work of Fabricius 2011 builds on earlier work off the island of Ischia, Italy; another area where CO2 bubbles up from the seafloor. Hall-Spencer (2008) reached similar conclusions researching the waters off Ischia; lower pH inhibited the development of calcifying marine life, and favoured the growth of seagrasses and slime. See video of the area, featuring Dr Hall-Spencer, below:
Ocean Acidification - Ischia from UNEP WCMC on Vimeo.
Future implications - oceans of slime?
The potentially devastating impact of future ocean acidification is clearly obvious in the pictures above. Despite being exposed to acidic conditions for thousands of years, it seems many marine creatures can't handle ocean acidification, and the ones that do are not conducive to healthy coastal ecosystems. The loss of of cructose coralline algae is particularly important to corals, as they secrete chemicals that provide settlement cues for coral larvae, help consolidate the reef structure, and prevent the growth of slime. Without the complex structure and shelter that healthy reefs provide, many thousands of reef species, including many fish, may disappear.
Fabricius 2011 adds to the growing body of scientific research which spell out a troubling future for our oceans. There will undoubtedly be some winners as the oceans become more acidic, but just how palatable is a menu consisting of slime, seagrass and brittlestars, and the occasional sea urchin? That is something future generations may have to to ponder.































 Arguments
Arguments






























[DB] IIRC, any reduction in seawater oxygen content due to the phytoplankton decline will be interspersed thoughout the various layers of the worlds oceans. That's a lot of volume. Given the volume of the atmosphere, any reductions in oxygen content due to that loss may have been offset by oxygen coming from melting ice sheets (Canadian Archipelago, Greenland, Antarctica as well as the global decline in alpince glaciation). Speaking off-the-cuff, as I haven't studied that particular aspect. Don't lose any sleep over it.
I have a question. I have heard that the oceans are under saturated by CaCO3 because the average concentration of Ca++ in the oceans is much lower than the sum of all carbonate, bicarbonate, and carbonic acid concentrations. However, when concerned with the solubility of the CaCO3 shells of living organisms, I would expect that the more relevant question is - is the sea water in the immediate vicinity of these living species saturated with respect to CaCO3? If so, one would observe a gradient of Ca++ concentration as one moves from the region of shelled species out towards the depths am remote regions of the oceans. Certainly such measurements have been made.
Note that Matt Ridley has suggested that increased atmospheric CO2 levels will actually facilitate the growth of CaCO3 shells (in his misnamed book, Realistic Optimist) - an argument that has meaning only if the sea water in the vicinity of shelled critters is not saturated w.r.t. CaCO3.
Any insight on this point would be apprecited.
Sorry, I meant to say because" average concentration of Ca++ in the oceans is much HIGHER than the sum of all carbonate, bicarbonate, and carbonic acid concentrations." Thus according to Ridley, increasing the latter three would assist in CaCO3 formation.
(as we also know, of course, increased acidity serves to decrease the conc of carbonate ion relative to bicarbonate and carbonic acid - thus working against CaCO3 (s) formation - an important point not mentioned by Ridley).
Eric - It shouldn't surprise you to learn that Matt Ridley is wrong. The oceans are well saturated with calcium ions, so they are not a consideration. The concentration of calcium ions dissolved into the oceans only changes on geological timescales (typically millions of years) - hence the shift between aragonite and calcite seas over these long periods.
It seems for many marine life the concentration (activity) of carbonate ions is the key because carbonate ions serve as one of the building blocks of the calcium carbonate (chalk) shell/skeleton. One of the reactions that takes place when extra CO2 is dissolved into the oceans is:
Seawater currently favours the left-hand side of that equation, so adding CO2 to the oceans is actually decreasing the activity of carbonate ions, which in turn makes shell-building ever more energetically expensive. Decrease the carbonate ion concentration sufficiently (calcium carbonate undersaturation) and we end up with seawater that is physically corrosive to marine calcifiers.
This corrosiveness is already occurring in the waters of the American Pacific Northwest where oyster larvae are now largely unable to survive in the wild, because they dissolve (Barton [2012]). The Antarctic is also seeing highly corrosive surface water too. See the photo below of a pteropod (sea butterfly) which was caught (alive) several years ago: