SkS Analogy 17 - Lotteries, evaporation, and superstorms
Posted on 24 January 2019 by Evan, jg
Tag Line
Evaporation is like a lottery. Lottery winners finance our storms.
Elevator Statement
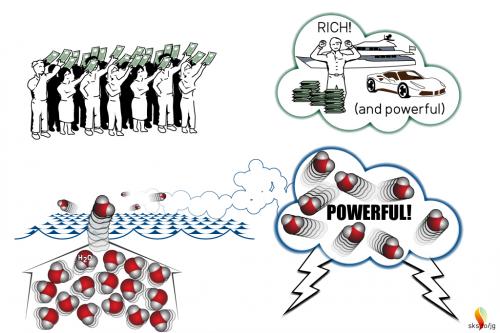
Each day 10,000 people each put $100 into a million-dollar lottery where winner takes all. Because lottery winnings are taxed, the winner receives $600,000 and the government receives $400,000. This leaves the people who bought the lottery tickets poorer, one person richer, and the government more powerful.
A puddle of water loses water due to evaporation. Evaporation occurs when many neighboring molecules share a little of their energy with a single molecule that gains enough energy to leave the puddle and fly away into the atmosphere. This molecule wins the lottery. The high energy required to send a single water molecule into the atmosphere causes the energy of the other molecules in the puddle to decrease (i.e., the temperature of the puddle decreases), while the amount of water vapor in the atmosphere increases. A fraction of the water vapor in the atmosphere is available to storms, so that as the total amount of water vapor in the atmosphere increases, the amount available to storms also increases (i.e., the fraction of a larger number means more is available). Because storms get their power from condensing water vapor, as more water vapor is added to the atmosphere more water vapor is available to storms, which increases their power. Therefore, evaporation leaves the puddle with less energy, the atmosphere with more water vapor, and storms with more power. This is how evaporation keeps Earth cool and supercharges storms.
Climate Science
For each 1°C increase in global temperatures the atmosphere holds about 7% more water vapor. It takes a lot of energy for 1 kg of water to go from the liquid to gas phase. With the energy it takes to vaporize 1 kg of water, you could heat 5.4 kg of water from 0 to 100°C! For perspective, if you weighed 150 lb, an extra 7% for each 1°C warming translates into the following weight gain.
Starting weight: 150
| 150 + 7% increase (1°C warming) | 161 |
| 161 + 7% increase (2°C warming) | 172 |
| 172 + 7% increase (3°C warming) | 184 |
| 184 + 7% increase (4°C warming) | 197 |
Think about how much energy it takes to vaporize an extra 7% more water vapor for each 1°C of global warming. And now all of that extra energy is floating around above your head, waiting to do the other thing that high-energy water vapor does well: supercharge storms. That is, the large amount of energy used to vaporize water is released when the water vapor condenses to the liquid phase in a cloud to form rain, snow, sleet, or hail. Higher-energy storms produce larger hail, stronger winds, more powerful tornadoes, and stronger hurricanes.
Here is another angle to the physics of evaporation. As noted, when water evaporates, overall the temperature of the system drops. By warming back up to the starting temperature, the system as a whole absorbs energy. There are at least three processes that allow Earth to absorb energy without any increase in air temperature:
- During a La-Nina cycle the temperature of the oceans can increase without increasing air temperature. Although subsequent El-Nino cycles dump the stored energy into the atmosphere, during a La-Nina cycle the temperature of the atmosphere can remain relatively stable or even decrease.
- Ice melts with no increase in the temperature of the local water-ice mixture.
- Water evaporates with a decrease in the temperature of the evaporating water.
The important point is that while each of these three processes occur, which do not result in the air temperature increasing, the total energy of the Earth continues to increase. Somehow, somewhere, at some time this extra energy will cause problems.
Read here for more information about the relationship between temperature, the latent heat of vaporization, and the energy of the atmosphere.































 Arguments
Arguments






























Very catchy, informative and entertaining analogy. I found myself trying to remember under what conditions evaporation occurs. It could be helpful to include an embedded link to a wikipedia article on evaporation, or a brief summary that evaporation can happen at any temperature above 0 degrees C, and is proportional to temperature, and the vapour pressure of the atmosphere etcetera. Or maybe this is superfluous to the thrust of the discussion, and over complicates it?
Several of the principles noted form the basis of evaporative coolers.
Another consequence of a warming world and higher levels of atmospheric moisture is storms produce more intense rainfall. This was very significant for Hurricane Harvey.
nigelj@1 Yes, evaporative coolers are a good example. When I was young we did not have air conditioning in our car. When travellig in the desert we put wet washclothes on our forehead to cool ourselves down. Same principle as an evaporative cooler.
Although evaporation only occurs above 0C, below that temperature the process is fundamentally the same, except it is called sublimation and not evaporation because frozen water is moving to the vapor phase. I enjoy watching a small pile of snow inside our shed in Minnesota slowly disappear in the middle of winter.
Dr. James Hansen: Ice Melt, Sea Level Rise and Superstorms Video Abstract
That was about the neatest description of evaporation and its consequenses I have read. Very nice. Note that latent heat from ice to water is only enough to raise the same amount of water from zero to 800C. Consequence..... If warm moist air flows accross Greenland, each kg of water that condenses out of the air releases enough heat to melt 5.4/8 = 6.75kg of ice. Makes you think.
Thanks for the feedback William. It's nice to know when analogies do/don't click. And your point about the melting of ice from condensation is important because these are the effects that people often don't think about, but which have large consequences. As you implicitly point out, it's not all about whether or not temperatures are going up.
Thanks for the video link SirCharles.
As this is your first post, Skeptical Science respectfully reminds you to please follow our comments policy. Thank You!
William @4: a typo. 5.4 / 0.8 = 6.75 kg of ice.