How substances in trace amounts can cause large effects
What the science says...
Small amounts of very active substances can cause large effects.
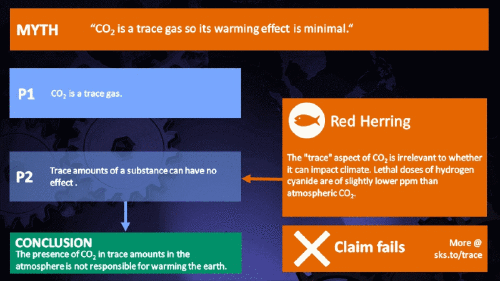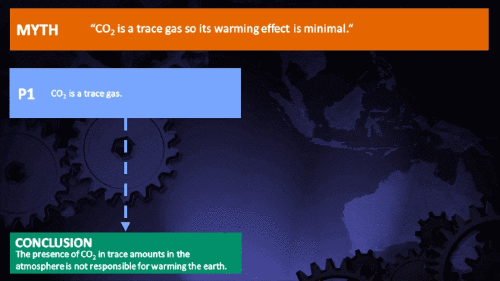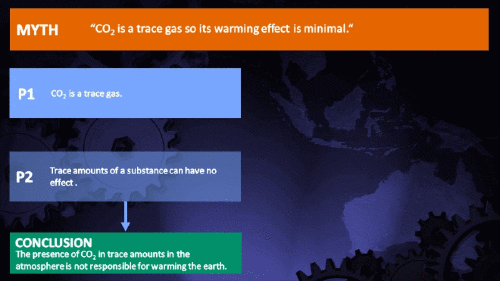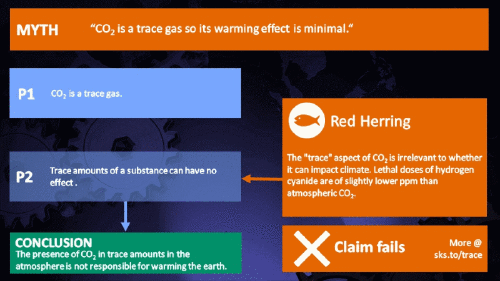
Climate Myth...
CO2 is just a trace gas
"We have been grossly misled to think there is tens of thousands of times as much CO2 as there is! Why has such important information been withheld from the public? If the public were aware that man-made CO2 is so incredibly small there would be very little belief in a climate disaster ..." (Gregg Thompson)
At a glance
When the first edition of this rebuttal was posted in August 2011, atmospheric CO2 was at around 390 parts per million (ppm). Now (August 2023) the number is 421 ppm - and rising.
To a non-chemist, 421 ppm might sound like a vanishingly small amount, simply because you're comparing a small number (421) with a very big one (1,000,000). It therefore comes as no surprise to find that this apparent contrast was seized upon by practitioners of misinformation. Claiming that things occuring in apparently tiny amounts must be harmless is such an easy talking-point to bandy about, since much of the intended audience is unlikely to deal with such numbers on an everyday basis. But it's also one great big red herring. Why?
Hundreds of parts per million may not sound like a lot, but in fact many substances have important properties when present at such levels. Here are a few examples:
- He wasn't driving drunk, he just had a trace of blood alcohol; 800 ppm (0.08%) is the limit in all 50 US states and limits are even lower in most other countries.
- Don't worry about your iron deficiency, iron is only 4.4 ppm of your body's atoms.
- That ibuprofen pill can't do you any good; it's only 3 ppm of your body weight (200 mg in 60 kg person).
- The Earth is only 3 ppm of the mass of the solar system.
- Your children can drink that water, it only contains a trace of arsenic (0.01 ppm is the WHO and US EPA limit).
- Ozone is only a trace gas: 0.1 ppm is the exposure limit established by the US National Institute for Occupational Safety and Health. The World Health Organization (WHO) recommends an ozone limit of 0.051 ppm.
- A few parts per million of ink can turn a bucket of water blue. The color is caused by the absorption of the yellow/red colors from sunlight, leaving the blue. Twice as much ink causes a much stronger color, even though the total amount is still only a trace relative to the water.
- Only 500 ppm of hydrogen sulphide (bad egg gas) in air is a hazardous level, as any health and safety fact-sheet will tell you. It will make you seriously unwell at that kind of concentration. In fact, at above 100 ppm, you can no longer smell the gas because its toxicity has switched off your sense of smell.
Just a trace-gas? Yeah, right.
It's not the concentration of a substance that necessarily matters. Instead, it's what any substance can do at a certain concentration and that property will of course vary from one substance to another. These are all useful things to recall when someone dismissively tells you that carbon dioxide is “only a trace gas”. It doesn't matter.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
Although percentage or parts per million are convenient ways to talk about the relative amounts of gases in the atmosphere, they only tell us how much there is compared to everything else up there. Concentration doesn’t give an absolute amount.
For example, most people will have trouble breathing on top of Mount Everest. The atmosphere still contains 21% oxygen, just like at sea level. But the air is rarefied, meaning the relative proportions of all of its constituent gases are the same but the density of their molecules has reduced by around two thirds. With every breath you take you will only get around a third of the oxygen molecules that you would at sea-level. It's the number of oxygen molecules that matter because through their chemical properties and interactions with our bodies, they support respiration - and therefore life.
We are now emitting some 44 thousand million tonnes of carbon dioxide every year (source: IPCC AR6, WG 3 Technical Summary 2022). Fourty four thousand million tonnes is a hard number to visualise. One way to do so is to consider that it's more than seventy times heavier than the combined weight of the current global human population. Maybe that's even more mind-boggling! But nitrogen makes up 78% of the atmosphere - it's vastly more abundant than CO2. Because of that, the concentration of CO2 - despite such vast emissions figures - just nudges up by a few ppm a year.
In 1959 the measured CO2 concentration was 316 ppm whereas at the time of writing (2023) it's 424 ppm. That represents an exponential increase of 108 ppm over 64 years - exponential because our annual emissions have now increased vastly, compared to the 1950s and 60s. That's despite the knowledge of the harm being done by them.
Pre-industrial levels of CO2 were around 280 ppm, so the overall increase to date has amounted to some 144 ppm - a 50% increase. That CO2 increase has in turn been sufficient to raise global temperatures by more than a degree celsius relative to pre-industrial times, so we have a bench-mark with which to look at future emissions. Furthermore, because CO2 is a non-condensing greenhouse gas, once it's up there in the atmosphere it stays around for a long time, so much of that 144 ppm increase still has work to do - meaning that the warming already caused by it will continue for many years.
Often we talk about a doubling of CO2 from pre-industrial levels (280 increasing to 560 ppm) and its effect upon temperature: Arrhenius did the first calculation on this over 100 years ago. He could not foretell a time when such a CO2 overloading might occur due to human activity, since the emissions of the very early 1900s were very low compared to now. We now have that doubling firmly in our sights. In the decade of 2013-2023, CO2 levels have risen by nearly 30 ppm. If our emissions flatlined at current rates, we would reach that doubling-point sometime in the 2060s - not that far off at all - and that's assuming there are no nasty surprises waiting for us in terms of feedbacks.
So we know the amount of CO2 in the atmosphere has increased in this manner because we have constantly measured its concentration since the late 1950s. We know from current and historical data that the climate has already significantly warmed,. The link between increasing greenhouse gases and increasing temperature is even clearer now than it was in the 19th Century. CO2 in our atmosphere is trapping energy that would otherwise escape to space. That's an intrinsic property of this particular substance.
If a trace gas can absorb and re-radiate IR at even low concentrations, what do you think will happen if we double that concentration? Twice as many CO2 molecules, all busy doing precisely what they do and for long into the future. CO2 may be a trace gas but because of that intrinsic property it has, that doesn't matter.
Cartoon summary

This Cranky Uncle cartoon depicts the "Red Herring" fallacy for which the climate myth "CO2 is just a trace gas" is a prime example. It is used to deliberately divert attention to an irrelevant point to distract from a more important point. Please see the accompanying blog post for more information about the cartoon collection.
Last updated on 13 August 2023 by John Mason. View Archives































 Arguments
Arguments






























Interesting.
So the major gases that make up our atmosphere are transparent to visible light. That means that light will not increase the temperature of those gases directly, doesn't it?
If visible light passes through a transparent atmosphere and reaches an opaque surface below, it can increase the temperature of that surface. In the absence of greenhouse gases, though, any energy reradiated out from the surface as infrared wouldn't do anything to raise the atmosphere's temperature either, right?
The atmosphere would still be heated when its particles collided with the surface, though, wouldn't they?
Rovinpiper @26 , yes the atmosphere would still gain heat from physical contact with the planet's surface.
The catch is, when you calculate out the effects of it all, you find you have an Earth surface which is well below freezing point of water. Earth would be a complete iceball. And that leads to the question: So what is the Goldilocks level for CO2 ? ( CO2 being the critically important Greenhouse gas, in the long run. )
Rovinpiper @26,
Just to be clear, @25 I was considering the transparency of the vast majority of the atmospheric content with respect solely to radiation from the Earth's surface. II made no mention of visible light or solar radiation.
Through the 'visible spectrum', the major components of the atmosphere are not entirely transparent. N2 & O2 will cause Raleigh Scattering (which is why the sky is blue) and O2 does have absorption bands within red light (as does water vapour).
If we consider 'solar radiation' rather than just the 'visible spectrum', the various Energy Balance diagrams show that 30% of 'solar radiation' is reflected back into space, 23% absorbed by the atmosphere and the remaining 47% absorbed by the surface. For comparison (so here measured as a percentage of total 'solar radiation'), the heating of the atmosphere by conduction is 5%, by evaporation 23% and by the absorption by GHGs 110% with just 6% of the Earth's radiation making it into space without spending time heating the atmosphere.
In the absence of any GHGs or water, conduction from the surface would remain although an atmosphere that cannot absorb radiation also cannot emit it so the surface air temperature will not necessarily be colder relative to the surface (which will be colder). Also considering the continued Raleigh Scattering and O2 absorption (but not the O3 absorption) suggests the majority (perhaps three-quarters) of the 23% sunlight absorption in the atmosphere would also remain.
I see. It is always a bit more complicated than I realize.
You are right, of course. You didn't say that O2, N2, and Ar are transparent to visible light. That was the understanding that I brought to this discussion.
I don't entirely follow what you are saying about the components of the heating of the atmosphere.
Conduction, I think, would just be heat transfer by contact between surface and atmosphere. That makes sense.
Heating by evaporation, I guess, water from the surface gets heated, vaporizes, and moves into the atmosphere carrying heat with it. Simple enough.
We already discussed how GHGs absorb and reemit radiation of certain wavelengths, but you've cited a confusing figure for this component. The amount of heat added to the atmosphere by energy absorbed by greenhouse gases is equal to 110% of the total solar radiation reaching the Earth?
How is that possible? Is it because energy can be absorbed and reemitted several times before finally escaping into space?
Would that total solar radiation figure that we are comparing things to include the 30% that is reflected or not?
Thanks for all of the answers. This is really interesting.
Rovinpiper - Some time ago I ran through the numbers on this. CO2 takes about 10^-6 seconds to emit excess energy as infrared radiation. At sea level each air molecule collides with another 10^9 times. This means than an excited GHG molecule will undergo 1000 collisions before it's statistially likely to emit, meaning that yes, the atmosphere as a whole is warmed by GHG absorption, and the emissions are due to the statistical emissions of the air mass as a whole.
And now a (very) brief explanation of how this works:
The rest of the equation is tied to the lapse rate, the rate of which the air is cooler with rising altitude, and the statistical likelyhood of an IR emission escaping to the space. The absorption and emission of energy repeats throughout the atmosphere until GHGs decrease with pressure to the point that 50% or more of the IR escapes to space, which is where convection stops. This is the tropopause, the separation between the convective troposphere and the static stratosphere.
The emission rate is determined by temperature, and the lapse rate (about 6.5C/km, varies widely with humidity, temps, etc) means that the emitting gases at the tropopause are cooler than the earths surface. Very importantly, changing the GHG concentrations changes that altitude. And that change in altitude means that there is an imbalance between incoming and outgoing energies until the entire atmospheric column to the tropopause has warmed or cooled to match incoming energy.
Global Warming Linked To Increase In Tropopause Height
So the surface is hotter than the tropopause, linearly by altitude, the tropopause emissions have to match incoming solar energy to stabilize, and our emissions have raised the tropopause. We're therefore warming.
"The amount of heat added to the atmosphere by energy absorbed by greenhouse gases is equal to 110% of the total solar radiation reaching the Earth?" The diagram is not saying heat is added to the atmosphere. It is showing the flows of radiative energy. The re-radiation induced by the GHGs is creating the extra flow. Remember that the radiation is directly measured. Radiation hitting surface is higher than that at TOA. It would have been be a head-scratch if we hadnt discovered the GHE. The key give-away is the spectrum of the incoming radiation.
Rovinpiper @29,
The percentages are of the the 341Wm^-2 total solar input, so includes that 30% reflected sunlight.
The 110% isn't because the radiation takes many steps to negotiate a path from the surface & out to space. It is because many such journeys don't make it to space but end up back on the surface. A gas emitting radiation does so in any direction, up, down or sideways. For a solid there is more directionality as it always has to be out and away from the solid surface.
To add a couple of points (or to sharpen them) from #30&31, the radiation induces a waggle in GHG molecules and such induced-waggles can result in radiation being re-emitted but that is very unlikely. It is almost certain that a waggling GHG molecule will collide with another air molecule in which the waggle is converted into thermal gas energy.[ I'm not sure the 10^-6s & 10^-9s @30 is entirely correct. The values are usually very well buried within the literature but values I've seen are more 10^02s & 10^-6s, that's hundreths of seconds & milliseconds.]
But importantly, such collisions between air mollecules can also induce those same waggles in GHG molecules. This is a far more common form of waggle-inducement and so it is the speed of the gas molecules, gas temperature, that determines how frequent such waggles are induced. These waggles too can emit radiation and being far more common are the mechanism which causes in the vast majority of GHG radiation. As it is temperature-dependent and atmospheric temperature drops up to the tropopause (12km up), the amount of such emitted radiation shooting round the atmosphere will reduce with altitude.
Adding 50% to the CO2 in the atmosphere means a photon has a shorter length to go before it hits a GHG but this does not of itself affect temperature. What is of paramount importance is the extra 50% increases the altitude at which this radiation has a clear shot at space. As this is almost always an altitude below the tropopause, the exra GHG results in a the GHGs shooting out into space being at a lower temperature than previous and thus reduces the amount of radiation lost to space. And this loss of coolling warms the planet.
I see.
So, tell me. Doesn't the spectrum of emitted radiation depend on the temperature of the emitting particle?
If that is the case, then doesn't it provide a pretty convenient test of this theory?
Not sure that "emitting particle" is right, but radiation spectrum is absolutely dependent on temperature. And, yes, you can use the theory to predict spectrum of radiation at TOA or at surface of earth, or by how much the spectrum should change if you increase say CO2 from 400 to 440ppm. These have all been done (eg here) and predictions match observations with exquisite accuracy (a fair bit of advanced tech depend on these equations being correct).
For this topic, the arguments I see from friends revolve around "it's only .04% of the atmosphere". I'd like a simplified argument for them related to how thick the troposphere is and how small the air molecules are.
I don't know how to do this, but perhaps one of you can...
If we look at the "million parts" of air as a single layer of molecules, how thick would it be on average?
Given the avaerage size of the molecules, if they are in one layer, what would be the area of that layer?
(there would have to be some assumptions on air pressue and humidity, changing air pressure with altitude, and the vacuum space between molecules I presume).
So the result I'm looking for is something like
"based on [assuptions specified], a single layer of air molecules would be on average xx nanometers thick, and cover an area of y.yy sq meters. In the ~12km of tropshere a photon would have to travel through zzz million (billion?) layers. So while the trace concentrations are low, travelling through zzz million layers over a 12km thickness drastically increases the possibility of encountering CO2 molecules."
It's kind of like the visability you experience on a clear day vrs a rainy (or smoggy, or foggy) day. Over a short distance, I can see my hand fine, over a longer distance my visibility is reduced even though the percentage of raindrops in the air is low, and I cant see islands/mountains which are only kms away.
Am I making any sense?
(another angle would be to have a single stack of air molecules 12km high... how many would there be?)
I am new to this site and trying to learn. Plese forgive me for taking such an elementary tack and guide me in the right direction if you can.
Has anyone seen the results of a simple green house experiment?
Problem: What is the temperatrure affect of Sunlight on volumes of air with different concentrations of CO2?
Thank you,
Bruce
Bruce 47. One issue is that the "greenhouse" effect is inapprobiately named. It doesnt work like a greenhouse which makes "simple" experiments interesting depending on you what are trying to measure. In a lab, you can measure the absorption of sunlight by increasing the CO2 concentration. However, you would quickly conclude as Angstrom did, that you can saturate the effect. However, in the real structure of the atmosphere, temperature and pressure varies with height and as a result you cannot saturate the effect. An experiment to demonstrate this would somewhat large...
However, the change in radiation as CO2 increases can be measured though it is not a simple design. See here for details. More indirect measures of the change have been done by both measuring change at surface (eg Evans 2006) or to outgoing radiation from space (eg Harries 2001).
Fixitsan @97 elsewhere,
You say "I struggle to find anyone who can offer a sensible explanation why it is, that if 0.04% of the atmosphere consisting of CO2 traps significant heat, enough to warm the planet an estimated 1 Celsius in 1 century, why is CO2 at higher concentrations not used more often (or even ever) in common or garden insulation."
99.95% of the Earth's dry atmosphere comprises N2, O2 or Ar but they are transparent to IR. It requires a more complicated molecule to absorb or emit IR at the temperatures found in the Earth's atmosphere. So any IR passing through the atmosphere will only be absorbed by those more-complicated molecules and IR will only be emitted these same molecules.
And this is only at certain wavelengths which equate to the various wobbles that can be induced in those molecules. Of these, in the dry atmosphere, the big daddy of the IR-reacting molecules is CO2 which acts at 15 microns wavelength (666 cm^-1 wavenumber). This effect is responsible for a big bite seen in the spectrum of IR emitted out into space.

Thus about 20% of the Earthly IR has to negotiate the CO2 in the atmosphere and this mechanism directly provides perhaps 7ºC of the full 33ºC pre-ndustrial greenhouse effect.
The important variable is the altitude at which the CO2 emits the 15 micron IR out into space, and specifically the temperature of that altitude. The hotter it is, the more IR is lost to space, helping to cool the planet. But a colder gases emits less and that then insulates the planet better.
Now, if the upper atmosphere at the altitudes at which CO2 allows this 15 micron IR to escape into space were really really really cold, you could double that direct 7ºC CO2 effect by blocking all the IR in that band. But you need that really really really cold temperature to achieve it.
So if you;re after "common or garden insulation", if you want to keep something warm by half-a-dozen degrees or more, it is far easier covering it with a more conventional insulating barrier.
As far as anthropogenic global warming goes, filling the atmosphere with extra CO2 concentrations results in the space-bound IR in the 15 micron waveband being emitted at higher altitudes and, because those higher altitudes have a lower temperature, less IR will this be emitted in the 15 micron waveband out into space adding to the insulating greenhouse effect.
Fixitsan, your instinct that 400 ppm is a small number is not helping you understand it's actual effect. Perhaps you need to also need realize that avagadro's number is very large.
Without looking it up, what does your instinct tell you about how far an infrared photon of appropriate wavelength would travel on average before interacting with a CO2 molecule (mean path length) for an atmosphere with 400ppm?
Now you can look it up...
You do not form accurate beliefs about the nature of reality from handwavy arguments that suit what you would like to believe. Scientists make as precise as possible predictions from theory and then make observations to check. The observed spectrum of infrared, whether measured at top of atmosphere by satellite or on earth surface match the predictions of greenhouse gas theory to a very high degree.
scaddenp, from taking your advice to look it up (and to double check my existing understanding) 15 micron infrared is 'thermal', or 'far infrared'
CO2 interactions at these wavelengths are considerable, or in other words CO2's energy absorption rate is highest with this far infrared, as opposed to near infrared, which is more like light and has no heating component, as far as humans being able to feel it is concerned (think infrared emitters in remote controls, cold to the touch)
What befuddles, is the fact that nowhere on earth at ground level has the science, the wit of man, or nature made use of this interactive property of CO2 with regards to the huge amounts of far infrared which is in among the built environment. From domestic appliances, to industrial applications, far infrared is abundant on earth's surface
Far infrared is also expensive, and hence why we choose to insulate with great care the manmade applications, generators or soaks of far IR in order to improve the efficiency of the gas, electricity, or solar derived application of IR.
Take a steel furnace for example, heavily insulated, you can imagine the amount of IR which could be lost without insulation. A domestic oven is barely different, and indeed, our homes are heated with far IR and we do our best to trap as much of that heat in as possible in order to keep the heating bills down. We do this with insulation, in the cavities between the external structural walls, in the double glazing, and even triple glazed windows, in the loft space and also under the floor.
Currently, most insulation contributes to heat storage by entrapping air in small volumes in a structural support, such as foams. We use foam boards, foam pellets, liquid expanding foams and so on, filled with air in many cases, in the small bubbles in the foam.
In double glazing we use Argon now, previously we used a vaccuum for example.
But, what we never use, is CO2. Yet, we see CO2 being portrayed as an immensely effective molecule which is purported to trap heat. That's an amazing property for any portable colourless gas to have. It has a lower thermal conductivity than Argon and would be batter in double glazing for that reason alone, and add into the equation the fact that it has an additional benefit over Argon that it traps long wave IR which we need to keep in our homes I am left wondering how it is we simply don't bother.
Argon is 23 times more plentiful in the atmosphere than CO2, perhaps that is reason enough to use it, but it is more expensive too.
Have we not reached the stage of thought yet where we can imagine that using the extremely strongly purported far IR heat trapping effects of CO2 in insulation down here at ground level, or, is there just something about CO2 which means it only traps heat in low pressure environments such as in the upper layers of the atmosphere ?
CO2 traps heat, but the wit of man has so far failed to put such an amazing property to any use on earth. How come ?
[BL] This is all pointless, irrelevant, and incorrect rambling.
Fixitsan @40,
A TV remote "has no heating component, as far as humans being able to feel" because it operates using just a couple of AAA batteries and, like the rest of the electro-magnetic spectrum, the wavelength used (0.94 microns) does indeed have a "heating component." And if you turn up the power, human beings will feel it.
Fixitsan @40 : you have made a fundamental error.
The wit of man is not the wit of a sealion.
Indeed, few sealions are witty. If any. Such is the nature of the beast.
Ha ho and hee haw...the humour is , well, it just about qualifies as humour
MA Rodger the battery size is irrelvant to all but a pedant. but regardless of that the AAA battery can easily generate huge heat if applied to a low enough resistance where IR will pour forth. I accept there is current limiting in the form of a resistor in series with the led to protect it, but the wavelength quoted of 0.94 microns is not the same wavelength already quoted as being of concern which is 15 microns. (I actually think I used that as a reerence because it was in the wording of one of the links I have been sent by this forum's members, maybe it was even NASA, although that's unimportant now)
So back to the 15 micron wavelength IR , of which there is a lot in every home and factory and yet, the existence of any CO2 based heat trapping device or construction or insulation is completely absent in society.
Am I also correct in assuming there is no repeatable experiment which can be performed in a lab which proves the heat trapping quality of CO2 or is there an absence of such an experiment because CO2 possesses that quality only as a direct consequence of it's lower pressure, at high altitudes ? (But that seems unlikely, no ?)
I guess the question I have is, can it be proven beyond doubt that CO2 traps heat at all ?
[BL] Skeptical Science is not place to to post random, irrelevant speculation.
Rather than choosing to follow the Comments Policy, Fixitsan has chosen a path that does not include continued posting here.
A new footnote was added to this rebuttal with a Myth Deconstruction as an animated GIF which is potentially helpful to quickly debunk this claim on social media.
I had this idea to pack some extra punch into the "ink" analogy.
If you use ink to color a candle, you may in fact experimentally verify that a dark colored one melts in the sun, while a lighter one stands firm.
This may be a fun experiment to educate children, but also to sway doubters that rely solely on "common sense" to form opinions.
Described in this twitter thread.
jgillis:
The photos of the different blue dye concentrations in your twitter feed are illuminating. Or should I say, absorbing. Or, oh, heck - radiation terminology can be so complex....
I also used dye to illustrate how concentration affects absorption in this "from the email bag" posting a year and a half ago. In addition to showing the effect of small concentrations, it illustrates how it is really the absolute amount of dye that is important - not the concentration.
https://skepticalscience.com/from-email-bag-beer-lambert.html
Please note: this rebuttal has been updated on August 13, 2023 and now includes an "at a glance“ section at the top. To learn more about these updates and how you can help with evaluating their effectiveness, please check out the accompanying blog post @ https://sks.to/at-a-glance
This is a very weak article, here’s why:
• You don’t use trace amounts of blood alcohol to trap a significant amount of heat
• You don’t use trace amounts of iron to trap a significant amount of heat
• You don’t use trace amounts of ibuprofen to trap a significant amount of heat
• You don’t use trace amounts of Earth to trap a significant amount of heat in the solar system
• You don’t use trace amounts of arsenic to trap a significant amount of heat
• You don’t use trace amounts of ozone to trap a significant amount of heat
• You don’t use trace amounts of hydrogen sulphide to trap a significant amount of heat
You might be able to use a trace amount of colour to trap a significant amount of heat, but here’s the thing, CO2 is colourless.
@48: full marks for missing the point!
@49: Perhaps you should have full marks for missing the point!