How substances in trace amounts can cause large effects
What the science says...
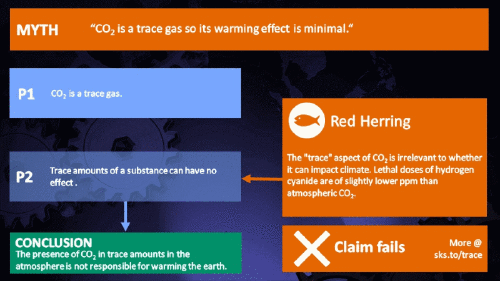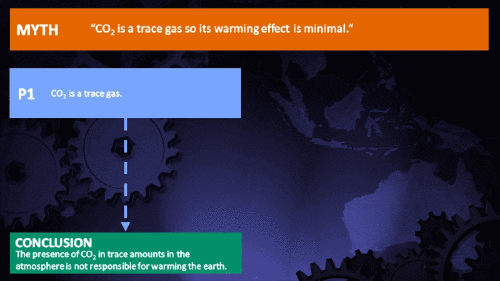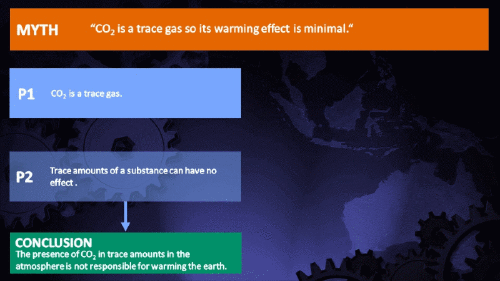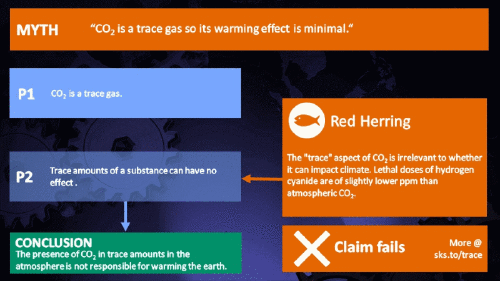
Small amounts of very active substances can cause large effects.
Climate Myth...
CO2 is just a trace gas
"We have been grossly misled to think there is tens of thousands of times as much CO2 as there is! Why has such important information been withheld from the public? If the public were aware that man-made CO2 is so incredibly small there would be very little belief in a climate disaster ..." (Gregg Thompson)
At a glance
When the first edition of this rebuttal was posted in August 2011, atmospheric CO2 was at around 390 parts per million (ppm). Now (August 2023) the number is 421 ppm - and rising.
To a non-chemist, 421 ppm might sound like a vanishingly small amount, simply because you're comparing a small number (421) with a very big one (1,000,000). It therefore comes as no surprise to find that this apparent contrast was seized upon by practitioners of misinformation. Claiming that things occuring in apparently tiny amounts must be harmless is such an easy talking-point to bandy about, since much of the intended audience is unlikely to deal with such numbers on an everyday basis. But it's also one great big red herring. Why?
Hundreds of parts per million may not sound like a lot, but in fact many substances have important properties when present at such levels. Here are a few examples:
- He wasn't driving drunk, he just had a trace of blood alcohol; 800 ppm (0.08%) is the limit in all 50 US states and limits are even lower in most other countries.
- Don't worry about your iron deficiency, iron is only 4.4 ppm of your body's atoms.
- That ibuprofen pill can't do you any good; it's only 3 ppm of your body weight (200 mg in 60 kg person).
- The Earth is only 3 ppm of the mass of the solar system.
- Your children can drink that water, it only contains a trace of arsenic (0.01 ppm is the WHO and US EPA limit).
- Ozone is only a trace gas: 0.1 ppm is the exposure limit established by the US National Institute for Occupational Safety and Health. The World Health Organization (WHO) recommends an ozone limit of 0.051 ppm.
- A few parts per million of ink can turn a bucket of water blue. The color is caused by the absorption of the yellow/red colors from sunlight, leaving the blue. Twice as much ink causes a much stronger color, even though the total amount is still only a trace relative to the water.
- Only 500 ppm of hydrogen sulphide (bad egg gas) in air is a hazardous level, as any health and safety fact-sheet will tell you. It will make you seriously unwell at that kind of concentration. In fact, at above 100 ppm, you can no longer smell the gas because its toxicity has switched off your sense of smell.
Just a trace-gas? Yeah, right.
It's not the concentration of a substance that necessarily matters. Instead, it's what any substance can do at a certain concentration and that property will of course vary from one substance to another. These are all useful things to recall when someone dismissively tells you that carbon dioxide is “only a trace gas”. It doesn't matter.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
Although percentage or parts per million are convenient ways to talk about the relative amounts of gases in the atmosphere, they only tell us how much there is compared to everything else up there. Concentration doesn’t give an absolute amount.
For example, most people will have trouble breathing on top of Mount Everest. The atmosphere still contains 21% oxygen, just like at sea level. But the air is rarefied, meaning the relative proportions of all of its constituent gases are the same but the density of their molecules has reduced by around two thirds. With every breath you take you will only get around a third of the oxygen molecules that you would at sea-level. It's the number of oxygen molecules that matter because through their chemical properties and interactions with our bodies, they support respiration - and therefore life.
We are now emitting some 44 thousand million tonnes of carbon dioxide every year (source: IPCC AR6, WG 3 Technical Summary 2022). Fourty four thousand million tonnes is a hard number to visualise. One way to do so is to consider that it's more than seventy times heavier than the combined weight of the current global human population. Maybe that's even more mind-boggling! But nitrogen makes up 78% of the atmosphere - it's vastly more abundant than CO2. Because of that, the concentration of CO2 - despite such vast emissions figures - just nudges up by a few ppm a year.
In 1959 the measured CO2 concentration was 316 ppm whereas at the time of writing (2023) it's 424 ppm. That represents an exponential increase of 108 ppm over 64 years - exponential because our annual emissions have now increased vastly, compared to the 1950s and 60s. That's despite the knowledge of the harm being done by them.
Pre-industrial levels of CO2 were around 280 ppm, so the overall increase to date has amounted to some 144 ppm - a 50% increase. That CO2 increase has in turn been sufficient to raise global temperatures by more than a degree celsius relative to pre-industrial times, so we have a bench-mark with which to look at future emissions. Furthermore, because CO2 is a non-condensing greenhouse gas, once it's up there in the atmosphere it stays around for a long time, so much of that 144 ppm increase still has work to do - meaning that the warming already caused by it will continue for many years.
Often we talk about a doubling of CO2 from pre-industrial levels (280 increasing to 560 ppm) and its effect upon temperature: Arrhenius did the first calculation on this over 100 years ago. He could not foretell a time when such a CO2 overloading might occur due to human activity, since the emissions of the very early 1900s were very low compared to now. We now have that doubling firmly in our sights. In the decade of 2013-2023, CO2 levels have risen by nearly 30 ppm. If our emissions flatlined at current rates, we would reach that doubling-point sometime in the 2060s - not that far off at all - and that's assuming there are no nasty surprises waiting for us in terms of feedbacks.
So we know the amount of CO2 in the atmosphere has increased in this manner because we have constantly measured its concentration since the late 1950s. We know from current and historical data that the climate has already significantly warmed,. The link between increasing greenhouse gases and increasing temperature is even clearer now than it was in the 19th Century. CO2 in our atmosphere is trapping energy that would otherwise escape to space. That's an intrinsic property of this particular substance.
If a trace gas can absorb and re-radiate IR at even low concentrations, what do you think will happen if we double that concentration? Twice as many CO2 molecules, all busy doing precisely what they do and for long into the future. CO2 may be a trace gas but because of that intrinsic property it has, that doesn't matter.
Cartoon summary

This Cranky Uncle cartoon depicts the "Red Herring" fallacy for which the climate myth "CO2 is just a trace gas" is a prime example. It is used to deliberately divert attention to an irrelevant point to distract from a more important point. Please see the accompanying blog post for more information about the cartoon collection.
Last updated on 13 August 2023 by John Mason. View Archives































 Arguments
Arguments






























JJones1960 even misses his own point.
CO2 is not "colourless" when it comes to infrared radiation. Just because JJones1960 can't see it doesn't mean it doesn't happen.
JJones1960 @48,
I hope the following helps you understand that John and Bob have correctly pointed out that you have made a very weak counter-presentation regarding the significance of small amounts. The points presented in the Argument effectively counter the simplistic and understandably incorrect belief that the percentage of CO2 in the atmosphere is too small to make a difference.
A major weakness of your counter-presentation is that you appear to lack even a small amount of knowledge regarding the matter, here’s why:
You stated • You don’t use trace amounts of ozone to trap a significant amount of heat
That belief is contradicted by improved evidence-based understanding (contradicted by learning what is already known). One of the many presentations about the global surface temperature impacts of ozone is the NASA Aura item: The greenhouse effect of tropospheric ozone. It opens with the following:
Tropospheric ozone (O3) is the third most important anthropogenic greenhouse gas after carbon dioxide (CO2) and methane (CH4). Ozone absorbs infrared radiation (heat) from the Earth's surface, reducing the amount of radiation that escapes to space.
A lot can be learned from the items presented on SkS and other reliable information sources.
Learning from reliable sources can make a world of difference.
In addition to what OPOF says about ozone, it should be noted that ozone in the stratosphere is an important absorber of UV radiation as well. Not that absorption of UV radiation in the stratosphere causes any noticeable heating. Oh, wait. It does.
As for the errors in using % or ppm as a measure of CO2 quantities, I'll beat my own drum and point to this blog post from a couple of years ago.
Bob Loblaw @ 51:
“CO2 is not "colourless" when it comes to infrared radiation. Just because JJones1960 can't see it doesn't mean it doesn't happen.”
The point that you miss that that CO2 is a trace gas, therefore cannot trap a significant amount of heat anyway.
OPOF @52:
Your quote:
“Tropospheric ozone (O3) is the third most important anthropogenic greenhouse gas after carbon dioxide (CO2) and methane (CH4).“
The point you miss is that ozone traps heat in relation to CO2 and methane as the ‘third most important greenhouse gas’ but that is IN RELATION to those gases. My point is that those gasses don’t and can’t trap a significant amount of heat because they are in trace amounts, therefore neither would ozone.
JJones @54 & prior :-
Your "trace" argument sounds a very useful one . . . useful in all sorts of situations ~ such as by the Flat-Earthers who will say: "The Earth must be Flat because [JJones] can only see a trace of surface curvature, from wherever he stands."
Or do I detect a trace of leg-pulling by you?
~ If so, then Congratulations [plus a trace of irony] .
jjones1960 @ 54:
After two months, the best you can come up with is an empty assertion that CO2 is a trace gas? On a post/thread that is devoted to demonstrating why that is such a bogus argument?
Well, let's review the calculation that you provide or reference, in support of your claim that CO2 "cannot trap a significant amount of heat anyway."
...oh, wait. You did not actually provide any calculation. Unfortunately for you, the people that have done the calculation come up with a different conclusion.
I miss the days when contrarians/deniers could actually put together a reasonable argument (however wrong) that presented some actual analysis (however wrong) that supported their positions. These days, it seems more and more that contrarians commenting here have nothing to say that goes beyond a 240-character slogan.
Re. #55 -
One man may unleash a catastrophic shooting. On 9-11, at least 19 men were involved. Compared to the global population of around 8 billion, that's a tiny percentage so by your reckoning they must have been harmless.
By JJones1960's reckoning I mean!
JJones - despite the examples in main article of very small amounts capable of having large effect, you seem to be clinging to idea that the concentration cant be important. Can we unpack this please? I want to see how you understand this?
From the basics, the sun warms the earth and heat is radiated out to space through the atmosphere as photons with wavelengths in the infrared part of the spectra.
Now as I understand it, you believe because the concentration is low, then there are not enough CO2 molecules to catch all the photons leaving the surface? Is that a reasonable summary of your position?
One way to check that sort of question is consider how far, on average, a photon at say 15microns wavelength might travel before hitting a CO2 molecule if the concentration of CO2 is 400ppm. If you want to think about it a very crude approximate way, then think of cylinder 15microns wide going to top of atmosphere. Now then what is chance of it encountering a CO2 molecule? Doing it properly is quite complicated because density of molecules varies with pressure as you go up the atmosphere, but can start with simple sealevel values and the gas equation.
If you start the calculation, eg how many CO2 molecules in a meter of that tube, then you immediately realise that while 400 molecules in a millions seems rather small, Avagadro's number is extremely large. There are a lot of CO2 molecules in the way.
In short, the photon will likely get only a metre or so before being captured. 400pm can easily trap all the photons in appropriate wavelength leaving the surface. To really understand the greenhouse effect though you have to know what happens next.
PS - you wouldnt walk into a room with 400ppm of cyanide gas would you?
scaddenp:
I previously pointed out in this comment on this thread that concentration in ppm is not a good way to determine the effects of CO2 on IR radiation. I stated that the absolute amount is the key, and pointed to this "from the email bag" post that illustrates this point. That comment was two comments above where JJones posted his first comment in March of this year, so it's probably too much to expect that JJones actually read it. He seems more interested in posting than in reading and learning.
As for JJones idea that CO2 in trace amounts can't absorb enough radiation, there are commercial CO2 gas analyzers that are designed to measure CO2 by measuring the amount of IR radiation it absorbs, and they can do this on very small quantities of air. One such instrument is described here:
https://www.licor.com/env/products/gas-analysis/LI-830-LI-850/
From the "how do they work?" section of that web page:
I expect that the manufacturer of this device (and the many manufacturers of similar devices) will be awfully disappointed to find out that they can't possible work, because JJones has asserted that trace amounts of CO2 can't absorb enough IR radiation to make a difference.
Bob, I agree but not many deniers are in the habit of respecting empircal tests over their biases and mental models. If they did, then deniers wouldnt exist. I am interested in how people build their mental models, and how we update these mental models as new information is presented. I am interested in just how JJones arrived at such a firm belief.
Scaddenp @61 : "I am interested in how people build up their mental models, and how we update these mental models as new information is presented."
By sheer chance, within the last few hours, I encountered a radio program discussing Conspiracy Theorists. One of the descriptors used was the narcissist personality of many conspiracists.
Epiphany. Had to kick myself, for not previously making the conscious link between narcissism and climate-denialism.
Sure, not all Denialists have rampant narcissism in their personalities ~ but many would have a slice of the "spectrum of narcissism" , manifest by: short-term thinking; selfishness and disregard of others; and of course motivated-reasoning to defend their climate-denialism. And presumably these traits also occur in the cynical paid-propagandists (e.g. Heartland propagandists, including the Emeritus types).
Put some table salt in your hand and dump out all but 2 grains. Keep hold of them while you read this:
Cyanide’s LD50, the dose that kills half those exposed to it, is 13 parts per million. Arsenic’s is 6. Some snake venoms, algal bloom toxin, & ricin, can kill at 1 part per million (0.0001%).
The main source of methyl mercury in the world is coal burning. It can kill in quantities smaller than 1ppm; at even smaller doses it doesn’t kill, it just has scores of profound lifelong physical and mental effects. Are there Alice in Wonderland fans here? Alice’s Hatter spoke in the bizarre tangles called word salad as a result of what’s Put some table salt in your hand and dump out all but 2 grains. Keep hold of them while you read the next 2 paragraphs.
Cyanide’s LD50, the dose that kills half those exposed to it, is 13 parts per million. Arsenic’s is 6. Some snake venoms, algal bloom toxin, & ricin, can kill at 1 part per million (0.0001%).
The main source of methyl mercury in the world is coal burning. It can kill in quantities smaller than 1ppm; at even smaller doses it doesn’t kill, it just has scores of profound lifelong physical and mental effects. Are there Alice in Wonderland fans here? Alice’s Hatter spoke in the bizarre tangles called word salad as a result of what’s still called Mad Hatter syndrome. It takes even less than that 1ppm in the chronic doses hat makers breathed in from the mercury nitrate (Hg(NO₃)₂xH₂O) they used for about a century to toughen fur fibers, allowing them to matt together better for a firmer hat. The process was called carroting because Hg(NO₃)₂xH₂O is orange.
Phyllobates terribilis, Golden Dart Poison Frog, ‘Orange’ (Imagine a picture here)
Dart poison frogs' batrachotoxin's LD50 is 1/1000th of that (1mcg/kg or 1 part per billion), so if those 2 grains of salt in your hand were batrachotoxin, it's a coin flip whether it would be enough to kill you just by holding it, if you had even a tiny cut on your palm. Sorry, there’s no antidote, and you have about 9 minutes left. It would take 6 salt grains worth of VX, while Botulinum toxin kills at 1 thousandth that amount—1 nanogram, or 1 billionth of a gram/kg body weight. One part per trillion of it can kill.
9 ppm is the maximum indoor safe carbon monoxide level over 8 hours. 200 ppm or greater will cause physical symptoms and is fatal in hours. 800 ppm of CO or greater in the air is fatal within minutes.
Bob Loblaw @60: "As for JJones idea that CO2 in trace amounts can't absorb enough radiation, there are commercial CO2 gas analyzers that are designed to measure CO2 by measuring the amount of IR radiation it absorbs, and they can do this on very small quantities of air."
Indeed I've bought an air quality meter for about $10 online, which uses non-dispersive infra-red (filters) to detect carbon dioxide, formaldehyde and other volatile organic compounds. It takes a minute to literally warm up, and is of course nowhere near as accurate as the infra-red equipment scientists use to measure CO₂, but it will at least detect breath, poor ventilation and includes a hygrometer.
Whether actual hands-on experience of such things will help someone accept radiative physics is one question. And whether finding that kind of evidence against the most convenient rationalisation available changes wider world-view about who is responsible for climate change is a different one.
Scaddenp @61 : "I am interested in how people build up their mental models, and how we update these mental models as new information is presented."
(digression) So am I, although I often find it hard to persuade people to share their reasoning, particularly if they are quickly on the defensive. I think humans in general do some 'hill-climbing' in their professed beliefs, aiming at local maxima of practical, satisfactory narrative. New information may change the landscape, but people only move their position slightly, rather than doing the tiring cognitive work of re-evaluating the bigger picture. Hence why goalposts are moved and people rapidly move on to the next myth.
One thing I do find is quite common among contrarians in real life (besides an understandable but exaggerated distrust of authority that is a mirror image of acceptance of consensus) is a simplistic version of Popper's falsificationism. Here's apparent evidence why climate science is wrong, and that is enough to disprove it. (Some do then accrete other supporting arguments.) This mischaracterised epistemology is something to apply to any scientific 'hypothesis' that may have been painted as inconvenient or costing money or jeopardising worldview, but from my experience they use a more common-sense Bayesianism for everyday life.
To JJones @48, one could add that 'a significant amount of heat' is radiated from Sun to Earth and Earth to space. If the latter is mostly in the form of long-wave infra-red, and carbon dioxide absorbs long-wave infra-red, where does the 'heat energy' go? And then maybe explain emission layer displacement in simple terms.
I'm surprised that this argument is so low on the popularity list, 77 out of 200. Possibly it’s more common offline: meeting some contrarians at real-life events (stalls etc), it’s practically what opens the conversation when you are pegged as one of the climate-concerned. ‘I bet you can’t tell me the amount of carbon dioxide in the atmosphere.’
If you express an answer in parts per million, or perhaps as two million million tonnes, then they will want it converted to a percentage (even though most of the atmosphere is transparent to infra-red, and it’s the amount rather than proportion of greenhouse gases that determines the greenhouse effect). 0.043% sounds negligible somehow, perhaps because of common uses of percentages in polling or economics or pay rises. Without empirical knowledge of effects of a substance in a small proportion, people can fall back on what seems like a reasonable guess.
An underlying assumption by people stressing concentrations seems to be that if people knew CO₂ was ‘a trace gas’, they wouldn’t be concerned about climate change, and so the way most people can’t answer in percentage terms means that they are ignorant about the subject matter, or have been manipulated. (The conversation may then proceed to ‘life flourished in the Jurassic because of higher CO₂’ myth, about as accurate as One Million Years BC with Raquel Welch, or combine several misunderstandings into one sentence or question.)
So, supporting the large effects of trace substances argument, and as some people reject the ‘poison’ or ‘alcohol’ analogy as too indirect, I’d like to post this table. If comparisons across the electromagnetic spectrum are somehow valid, then 0.043% turns out to be a lot.