Recent Comments
Prev 1503 1504 1505 1506 1507 1508 1509 1510 1511 1512 1513 1514 1515 1516 1517 1518 Next
Comments 75501 to 75550:
-
dana1981 at 11:58 AM on 22 September 2011Lessons from Past Climate Predictions: IPCC AR4
Charlie, the climate model projections are quite clearly not linear (see Figure 2), so I don't know why you're projecting a linear trend out to 2100. As the post states, we digitized the IPCC's multi-model average for scenario A2 (the red line), and the short-term trend was simply calculated in Excel. As the post notes, it's too short of a timeframe to say anything meaningful. -
SkS Responses to Pielke Sr. Questions
To all participants - Personal opinion here, all caveats accepted. I would like to thank Dr. Pielke for participating - I wish he would have continued. While I strongly disagree with his viewpoints on any number of topics, I find that a conversation is the only way to understand where differing opinions come from, and the only way in many cases to move forward is equipped with that knowledge. While the conversation here occasionally became more heated than productive, I would have to say that I have learned quite a bit from all participants. But please - can we get back to discussing the science, rather than rhetorical "position statements"? And I most definitely include Dr. Pielke in that sentence! -
SkS Responses to Pielke Sr. Questions
Eric, discussion is impossible when one party misrepresents another. It is in effect a strawman. Misrepresentation must be clarified. -
SkS Responses to Pielke Sr. Questions
Eric (skeptic) - "Misrepresented is a false accusation. Dishonorable is an insult. Not worthy of this site." If (as has happened to me upon occasion) my statements are reworded to twist the meaning, that's definitely "misrepresentation". And I will continue to call folks on it when it occurs. I would, however, agree about the word "dishonorable". Discussions may be quite frustrating, and everyone will certainly form their opinions about the participants. While it is reasonable (if quite rude) to say that something 'seems like xxxx to me', it's quite unreasonable to assign such motives to another person. -
Eric (skeptic) at 10:49 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
#125, unfortunately getting into arguments about whether one is being misrepresented or not is almost always off topic and unproductive. It is far better to return to the original topic and repeat the question (clarify if possible) or repeat the answer and clarify that. -
michael sweet at 10:46 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Albatross, I rarely comment anymore because I have noticed that you and several other commenters do a better job than I do. I did not previously understand how detailed your knowledge of the state of the literature was until I saw this discussion. It is too bad that Dr. Pielke decided to dodge so many of your points. I can certainly make up my mind about the points that were made. I am especially stunned that Dr. Pielke would present his paper with 18 coauthors as peer reviewed and then provide no evidence of peer review when you quoted the journal as saying the item was not peer reviewed. That alone speaks volumes. If the paper was peer reviewed, why is the evidence not promptly provided? Your point that Dr Pielkes' attempt to argue from authority is countered by the position paper of the Union itself was perfect! Thank you for your succinct discussion of the detailed points that only an exceptionally informed person could make. Dr Pielke would have fooled me on many points without your clear refutation of his claims.Response:[DB] Albie is a big-leaguer and we at SkS are very fortunate to have him here as a Forum member and participant.
-
NewYorkJ at 10:44 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
When Person A asserts that Person B is misrepresenting Person A's argument or ignoring a question, and Person A provides evidence for that, Person B should either 1. Concede/retract (apology or not) or 2. Challenge Person A This is one way that debate moves forward and learning is accomplished. Sometimes it's an honest mistake or miscommunication on either/both persons involved. Taking a sincere interest in what someone else's argument helps, and there's no shame in conceding you might be wrong. By my observations, Dr. Pielke has chosen less constructive approaches here. However, I for one appreciate him taking the time to stray beyond the usual comfort zone blogs, many of which contain a level of uncivility far surpassing the worst seen here. -
Stephen Baines at 10:40 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
"Perhaps he's had a long day and is just tired" I actually thought this a benign comment, providing him with a clear out. I often lose a little patience late in the day. Not everyone is made for this kind of give and take. Too bad...I was starting to get a real impression of what his position was, which I've never quite understood before. Didn't quite get there though. I also thank him for coming on. -
Eric (skeptic) at 10:36 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Misrepresented is a false accusation. Dishonorable is an insult. Not worthy of this site. -
skywatcher at 10:20 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Dr Pielke, you seem to take offence rather too easily when somebody disagrees with your position. If all you ever refer to are your own papers (largely true in these exchanges), you very easily fall into the trap of "one-sided skepticism" by not considering the full body of literature. Perhaps you're just looking for a reason not to answer the many questions you have so far ducked in the various exchanges. I would note you've done exactly what VeryTallGuy suggested in #75, among his pertinet points in that comment. I remain absolutely astounded that somebody so interested in regional climate change seems not to think that CO2 and other long-lived greenhouse gases are the strongest positive global radiative forcing element (#94). You also appear to think that the warming forced by greenhouse gases cannot have a significant effect on a regional scale through influencing and changing weather patterns. Much of this is no longer in the model realm, as these changes are now observed. How on earth do you mitigate sea ice reduction (albedo), ocean acidification, sea level rise, or globally-forced weather pattern changes on a regional scale? -
Charlie A at 10:15 AM on 22 September 2011Lessons from Past Climate Predictions: IPCC AR4
You say "The model projections actually begin in 2000. The trends over that period are 0.12°C (projected) vs. 0.15°C (observed) per decade." Are we to understand that 0.12C/decade is the trend for the projection for A2 scenario from 2000 to 2010 ?? 0.12C/decade is obviously 1.2C/century, so it is easy to draw a straight line from the year 2000 point on the graph to a point at year 2100 that is 1.2C higher. That line falls well below the red A2 slope for 2000 to 2010. Your claim that the AR4 A2 projection is 0.12C/decade also disagrees with the text statement in AR4 that said there is little difference between the different scenarios for the first 20 years or so, and that the slope of all projections is about 0.2C/decade. Please clarify how you came up with 0.12C/decade. -
dhogaza at 10:02 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
"These types of non-constructive comments are a major reason I stopped accepting comments on my weblog." A willingness to apologize when one has misrepresented another's position is one of the most fundamental attributes of an honorable person. " Readers on your weblog who have not commented can make up their own minds on the exchange of views that have already occurred." I, for one, would like to thank RPSr for having confirmed my previous impression of him from years of having occasionally read his blog. -
pielkesr at 09:52 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
John Hartz - "Perhaps he's had a long day and is just tired" "Please let me remind you that it was you who chose to misrepresent my position and my statement on citations in your post @105, and at the same time belittle it. Now you are trying to take offense to the fact that I was annoyed/offended by you misrepresenting me and are trying to accuse me of being discourteous?" This type of exchange occurs too much on your weblog. These types of non-constructive comments are a major reason I stopped accepting comments on my weblog. Therefore, I appreciate you giving me an opportunity to express my views. Readers on your weblog who have not commented can make up their own minds on the exchange of views that have already occurred. However, I require a courteous exchange of viewpoints, even when there is disagreement, and, unfortunately, except for several excellent open-minded and cordial comments by your readers, a large fraction of the comments are not of that type. Much of my effort is going in circles and repeating myself. Thus, this is my last comment on your weblog.Moderator Response: [John Hartz] Que sera, sera. -
Albatross at 08:49 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
John @118, Me neither John. That post @ 117 by Dr. Pielke came out of left field.Moderator Response: [John Hartz] Perhaps he's had a long day and is just tired. -
Albatross at 08:32 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Dr. Pielke, Please let me remind you that it was you who chose to misrepresent my position and my statement on citations in your post @105, and at the same time belittle it. Now you are trying to take offense to the fact that I was annoyed/offended by you misrepresenting me and are trying to accuse me of being discourteous? Let me also not that earlier when I mistakenly misrepresented your position on an issue and I immediately apologized. And lastly, I am not the one who has had offensive statements snipped from their post on this thread, that was you. I am afraid that your reasoning for ceasing what had been an energetic exchange of ideas and science makes no sense. I'm sorry that you feel that way and feel that your indignance is not warranted. Feel free to ignore me, but I am still free to post comments and critique on your posts here.Moderator Response: [John Hartz] This is not how I envisioned tying off the discussion on climate models. -
Philippe Chantreau at 08:13 AM on 22 September 2011CERN - Saying Nothing About Cosmic Ray Effects on Climate
Thank you for finally providing some real references. However, no connection has been proven yet. One needs to look at more than one paper when assessing the state of knowledge about a particular issue. IF the effect is real, which is a big if, since there is still no physical mechanism for particle growth, it is minute, at best. That's according to Laken et al (2010), the very paper you linked. I note that an earlier Laken paper still couldn't find a correlation. Kulmala et al (2010) looked at various data over an entire solar cycle and failed to detect anything meaningful: "Our analysis shows that none of the quantities related to aerosol formation correlates with the cosmic ray-induced ionisation intensity (CRII)." Nothing is proven in the GCR/cloud "relationship." At best, it is controversial and if an effect does exists, it is very small. Laken et al (2010) concludes: "The climatic forcings resulting from such solar – terrestrial links may have had a significant impact on climate prior to the onset of anthropogenic warming." I find a strong discrepancy between this language and your interpretation. -
pielkesr at 08:06 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Albatross - I keep answering your questions. Also, with this kind of discourteous response "Please so not play word games with me, and please do not misrepresent and distort what I said, as well as ignore the reasons provided for my concerns. I suggest that you read my post again carefully and reflect.' I am finished responding to you.Moderator Response: [John Hartz] A casual observer might conclude that you are purposely ducking the issues posed by Albatorss in a very straightforward, non-confrontational manner in #115. -
Albatross at 07:37 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Sorry John Hartz, I was composing my most recent post and only saw your note after I posted. OK, moving on :) Besides, I have to take care of some stuff.Moderator Response: [John Hartz] Thank you. I know from expereince that "model-heads" can get into passionate and lengthy discussions about the subject. -
Albatross at 07:35 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Dr. Pielke @113, "If you conclude that in order to be a "first-order" climate forcing it must significantly alter the global annual average radiative forcing, than this conflicts with the NRC (2005) report." OK, let me clarify. Maybe I missed it, but I did not see the report refer to land-use change as a primary or first-order of global climate. They do say that: "The strengths of the traditional radiative forcing concept warrant its continued use in scientific investigations, climate change assessments, and policy applications. At the same time, its limitations call for using additional metrics that account more fully for the nonradiative effects of forcing, the spatial and temporal heterogeneity of forcing, and nonlinearities." They also say that: "Regional variations in radiative forcing are likely important for understanding regional and global climate responses; however, the relationship between the two is not well understood. Regional climate responses can also be caused by global forcings, making it difficult to disentangle the effects of regional and global forcings." And they include this figure from TAR: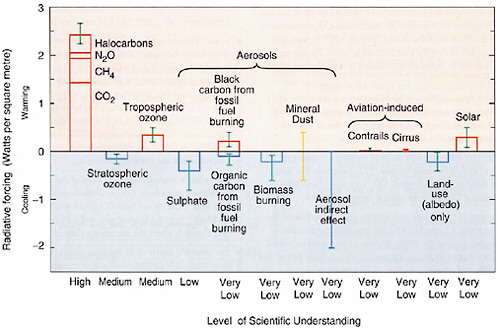 The NRC report says:
"The largest positive forcing (warming) in Figure ES-2 is from the increase of well-mixed greenhouse gases (CO2, nitrous oxide [N2O], methane [CH4], and chlorofluorocarbons [CFCs]) and amounted to 2.4 W m−2 (watts per square meter) between the years 1750 and 2000. Of the forcings shown in the figure, the radiative impact of aerosols is the greatest uncertainty."
Land albedo does not rank very high and Myhre and Myhre (2003) found that the albedo affect is probably <+/- 0.5 W m-2.
They do not that:
"The concept is inadequate for some forcing agents, such as absorbing aerosols and land-use changes, that may have regional climate impacts much greater than would be predicted from TOA radiative forcing."
Note they say inadequate, not inappropriate or unacceptable.
They recommend:
"The net radiative forcing of the atmosphere can be deduced from the difference between TOA and surface radiative forcing and may be able to provide information on expected changes in precipitation and vertical mixing. "
They note:
"Regional variations in radiative forcing may have important regional and global climatic implications that are not resolved by the concept of global mean radiative forcing."
Note they say "may".
That was 2005. If you object to spending money on decadel projections, fair enough you are entitled to your opinion, but then how do you reconcile that with urging governments to spend money investigating hypotheticals? You seem to be using this hypothesis to confuse policy makers in the USA into not mandating that prompt and meaningful action be taken on AGW. That may not be your intention, but that is certainly how they will perceive it if they are reluctant to move forward, you may be unwittingly (or not) providing them a convenient a sophisticated sounding excuse. It does not have to be an either or, we can address both, while also taking prompt action on reducing GHGs.
Moderator Response: [John Hartz] Excellent post!
The NRC report says:
"The largest positive forcing (warming) in Figure ES-2 is from the increase of well-mixed greenhouse gases (CO2, nitrous oxide [N2O], methane [CH4], and chlorofluorocarbons [CFCs]) and amounted to 2.4 W m−2 (watts per square meter) between the years 1750 and 2000. Of the forcings shown in the figure, the radiative impact of aerosols is the greatest uncertainty."
Land albedo does not rank very high and Myhre and Myhre (2003) found that the albedo affect is probably <+/- 0.5 W m-2.
They do not that:
"The concept is inadequate for some forcing agents, such as absorbing aerosols and land-use changes, that may have regional climate impacts much greater than would be predicted from TOA radiative forcing."
Note they say inadequate, not inappropriate or unacceptable.
They recommend:
"The net radiative forcing of the atmosphere can be deduced from the difference between TOA and surface radiative forcing and may be able to provide information on expected changes in precipitation and vertical mixing. "
They note:
"Regional variations in radiative forcing may have important regional and global climatic implications that are not resolved by the concept of global mean radiative forcing."
Note they say "may".
That was 2005. If you object to spending money on decadel projections, fair enough you are entitled to your opinion, but then how do you reconcile that with urging governments to spend money investigating hypotheticals? You seem to be using this hypothesis to confuse policy makers in the USA into not mandating that prompt and meaningful action be taken on AGW. That may not be your intention, but that is certainly how they will perceive it if they are reluctant to move forward, you may be unwittingly (or not) providing them a convenient a sophisticated sounding excuse. It does not have to be an either or, we can address both, while also taking prompt action on reducing GHGs.
Moderator Response: [John Hartz] Excellent post! -
tblakeslee at 07:29 AM on 22 September 2011CERN - Saying Nothing About Cosmic Ray Effects on Climate
The Forbrush event is like when a building collapses in a fire. It is part of the fire but not all of it. It is handy for proving the connection because it is abrupt. The activity of the sun varies with the predictable cycles and produces important but not as dramatic magnetic fields. Didn't you read the Laken paper? -
Albatross at 07:03 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Dr. Pielke, My apologies if I misrepresented your position-- that was my honest take. Your (non) apology for misrepresenting me is accepted ;) So you are suggesting that we do not bother with improving forecasts (despite users, policy makers, governments and stake holders demanding them of scientists) because of the complexities of the climate system are just too great? But let us differentiate carefully between choosing our battles with models and their development and tossing them out with the bath water as some suggest (Lindzen in particular has no time for models, and Specner has little interest in those models with higher climate sensitivity). "What do we know today from these models that we did not know in 1992?" I sincerely hope that this question made in jest. Can you clarify, in your testimony you said "There is no way to test hypotheses with the multi-decadal global climate model forecasts for decades from now as step 2, as a verification of the skill of these forecasts, is not possible until the decades pass." Yet here you are arguing that the models cannot predict decadal climate but at the same time you are telling others that there is no way of testing whether or not they can do so. But your hang up seem to be the time frame, well yes, that is an issue, but we do not need to wait further before taking meaningful action on GHGs. It would be foolhardy to argue that we need complete understanding (and high confidence and low uncertainty) in order to move forward. Uncertainty cuts both ways, just just towards lucky breaks and low climate sensitivity. And again, other lines of evidence such as the paleo record a indicate that it would be wise to be prudent and taking this matter very seriously. The science and models are advancing, for example the climate sensitivity NASA's GISS model has reduced slightly over the years as they have implemented changes. Advances have been made in computer power (which is getting cheaper) that has allowed finer grid spacing to be used, both in the vertical and horizontal, and also allowed the implementation of fully coupled AOGCMS and more sophisticated land-surface schemes, including dynamic vegetation schemes. Surely you can recognize that an immense amount of change has taken place in the modeling world since Manabe et al.'s (1991,1992) seminal work. Do you wish for that to cease? Or is is just the decadal projections that you are opposed to?Moderator Response: [John Hartz] Please try to wrap up your dialogue with Dr. Pielke on climate models. We need to move on to other items of discussion such as the appropriate metrics for measuring climate change. -
muoncounter at 06:57 AM on 22 September 2011CERN - Saying Nothing About Cosmic Ray Effects on Climate
tblakeslee#85: "Forbush events ... useful for proving the connection to cloud formation." Except that connection is not proven. Once again, Dragic finds very few FDs have any measurable effect. As proof, you offer a Svensmark paper. Not impressed. But then, in the same paragraph, you state "important driver of cloud cover is not the Forbush events but the predictable cycles driven by planetary positions." Which is it? You can't play it both ways: either FDs have an effect or they do not. If planetary cycles are the drivers, what does that have to do with any possible cosmic ray induced cloud formation? -
pielkesr at 06:48 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Albatross - On #102 You write Thank you. I think I know how we can come to terms on your position about land use and land cover change. I'm going to make a suggestion to you comment: "Land use-land cover change is a first order forcing for regional climate as it can alter regional climate more than that caused by the radiative forcing of added CO2; in contrast, the role Land use-land cover change in driving global climate change is still uncertain and as shown by Forster et al., is unlikely to be significant relative to forcing from GHGs and aerosols." On that I can agree. And I'm not sure how you can continue to argue that something which has (near) zero net global radiative forcing can be considered a first-order radiative forcing." If you conclude that in order to be a "first-order" climate forcing it must significantly alter the global annual average radiative forcing, than this conflicts with the NRC (2005) report. -
pielkesr at 06:38 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Albatross You write "Your proposed method of dealing with AGW sounds like it involved a lot of delay and even more research money over and above what is already being spent." This is completely contrary to my view. We are wasting vast amount of money on multi-decadal global climate predictions. I much prefer those funds be spent for technology development for more efficient energy sources including non-fossil fuel sources, and adaptation. We already know CO2 is a first order climate forcing. Everything else being done to provide more detailed forecasts on decadal time scales is a waste as we keep learning more of the complexity of the climate system and the challenges of skillful forecasts. What do we know today from these models that we did not know in 1992? On #103, I already answered John Hartz question in two comments. -
Albatross at 06:32 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Dr. Pielke @106, "So one is not permitted to refer to their peer reviewed papers to answer a question that the person would have known if they had read it" [inflammatory text snipped] Please do not misrepresent and distort what I said, as well as ignore the reasons provided for my concerns. I suggest that you read my post again carefullyand reflect. I obviously did not say that one should not permitted the peer-reviewed literature, in fact what you say is the opposite of my point. I very clearly spelled out why on must in fact DO that (i.e., consult the body of knowledge, understanding and science). People reading your comments on this thread would think that there are very few people working on land surface processes, and land-atmosphere feedbacks, and that you are one of those few. That is most certainly not the case. Or do you disagree with that fact?Moderator Response: Comment edited as requested. -
pielkesr at 06:31 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Albatross - Re #95, I posted on their paper. See http://pielkeclimatesci.wordpress.com/2011/08/30/new-paper-observed-changes-in-surface-atmospheric-energy-over-land-by-peterson-et-al-2011/ Their study found the same larger values as we did, as I dicuss in my post. -
pielkesr at 06:28 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
John Hartz - If the readers are not familiar with the common acronyms, they will not likely be familiar with what they mean.Moderator Response: [John Hartz] If acronyms are defined, the average person will have a better chance of understanding a statement. The golden rule of effective communication is, "Know thy audience." -
pielkesr at 06:27 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
John Hartz - If I have to define diabatic heating, [-snipped offensive comment-] Diabatic heating is a change of heat of a "parcel of air" in Joules/meter cubed that are distinct from changes in Joules per meter cubed due to expansion and compression (adiabatic heating) such as from ascent or decent.Moderator Response: Please do not make offensive statements about SkS or its readers, you were encouraged to post here and the Comments Policy applies to you too. -
John Hartz at 06:27 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Memo to all persons posting comments: The target audience for SkS articles and comment threads is the average person. Please define the acronyms that you use in your posts. -
Albatross at 06:23 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
John Hart, Sorry. Hope this helps. WRF is a regional numerical weather prediction model (NWP)used by the US National Weather Service and by researchers. AOGCMs are coupled atmosphere-ocean general circulation models. I'll note the "if" in Dr. Pielke's post @100-- this is an interesting hypothesis, but it is a hypothesis (versus the theory of AGW) and Dr. Pielke admits that it is his "view". So again, Occam's razor applies, we ought to be far more concerned about dealing with GHGs, aerosols and black carbon (I hope that you advocate moving away from coal rapidly). In fact research has looked into where we can get the biggest bang for our buck, and this matter has been discussed at RealClimate. Addressing land use change and CO2 emissions are not mutually exclusive, I know that you are familiar with REDD", you have made reference it in one of your papers. Your proposed method of dealing with AGW sounds like it involved a lot of delay and even more research money over and above what is already being spent. How would Roy Spencer feel about that? -
pielkesr at 06:23 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Albatross 'And I speak for my (and your) colleagues too when I say that it is considered poor form and inappropriate to repeatedly quote/cite one's own work as you seem to have a propensity to do." So one is not permitted to refer to their peer reviewed papers to answer a question that the person would have known if they had read it. Very unusual. :-) -
tblakeslee at 06:19 AM on 22 September 2011CERN - Saying Nothing About Cosmic Ray Effects on Climate
83 Forbush events are rare but useful for proving the connection to cloud formation. Here are two papers that confirmed the connection. Laken http://www.atmos-chem-phys.org/10/10941/2010/acp-10-10941-2010.pdf http://www.agu.org/pubs/crossref/2009/2009GL038429.shtml The important driver of cloud cover is not the Forbush events but the predictable cycles driven by planetary positions. The ENSO has a pattern that is related to these planetary positions. It is described by the Landscheidt paper whose link I posted. The paper was also published in 2003 in Energy and Environment. I referenced the blog version for your convenience. -
Eric (skeptic) at 05:49 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
It appears that progress can be made on narrow and specific issues, but those are fairly benign such as reducing GHGs. What is more important IMO is determining exactly where there are disagreements and spelling out those disagreements with as much precision as possible. A link may work in some cases, but trying to wade through a long blog post about why some paper is wrong makes it difficult to determine points of agreement and disagreement especially when that blog posting addresses many different points, comments on politics, etc. I would rather see the points of agreement or especially disagreement summarized here in concise bullet form. As just one example, could we have an estimate from Dr. Pielke of how much of the increase in nighttime minimum temperatures is due to station bias and how much is due to CO2 plus increased water vapor. I don't see a quantity, at least not in the 2009 paper. OTOH, I don't believe all biases are eliminated from the surface records particularly regarding impediments to radiational cooling in urban areas.Moderator Response: [John Hartz] You are correct. A linear comment thread like this does have its drawbacks. Having said that, let's complete the process of reviewing all the scientific issues raised in Dana's article. -
Albatross at 05:46 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Dr. Pielke could you please answer John Hartz's question posed to you @90 and again @95. Thank you. -
Albatross at 05:44 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Dr. Pielke @96, Thank you. I think I know how we can come to terms on your position about land use and land cover change. I'm going to make a suggestion to you comment: "Land use-land cover change is a first order forcing for regional climate as it can alter regional climate more than that caused by the radiative forcing of added CO2; in contrast, the role Land use-land cover change in driving global climate change is still uncertain and as shown by Forster et al., is unlikely to be significant relative to forcing from GHGs and aerosols." On that I can agree. And I'm not sure how you can continue to argue that something which has (near) zero net global radiative forcing can be considered a first-order radiative forcing. The forcing from CO2 is going to continue to escalate of course and the resulting changes will also impose feedbacks, included but not limited to land cover change... Re EOS, if someone were to contact them and ask whether or not your 2009 manuscript underwent official "peer-review", with peer-review as understood in terms scientific papers. What would they say? Also, do you honestly think that the government official to who you presented your 'paper' understand these nuances? "Also the alterations in spatial diabatic heating from this heterogenous forcing may alter large scale circulation features such as Asian monsoon, the NAO etc. " Earlier you referred to Takata as a example of regional change. Now you are using it as an example of large-scale circulation changes, while technically correct (the monsoon and AO is a large-scale phenomenon), that language may confuse readers. Also, the science has shown that oscillations (i.e., internal variability, do not account for the observed increase in global temperatures). See here, for example. And yes I have given your paper a cursory read-- iI have quoted from it above remember Have you watched Dr. Alley's talk yet? No models required to understand that we need to reduce GHGs aggressively and promptly ;) -
tblakeslee at 05:43 AM on 22 September 2011CERN - Saying Nothing About Cosmic Ray Effects on Climate
82 "Cosmic rays don't care where they land" but cloud formation can occur without cosmic rays when dust particles are present. There are clouds of dust over parts of the ocean but the oceans cover 2/3rds of the earth. Vast areas are dust free. Yes we have satellites to monitor fields but the standard idea of the sun as a fusion reactor ignores the electrical basis. The reference I gave clearly explains the flaws in the present sun theories. 81 Are you saying that the ocean temperature should change instantly as insolation changes? It is obvious that the ocean temperature changes slowly. Please read my citation if you think the sun is well understood. -
pielkesr at 05:32 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Dikran Marsupial - It would be much more effective if you would directly contact David Douglass and ask your question about his analysis. This would be another example of reaching out to seek to work together on the climate issue. I have respect for David and he will clarify or correct if their is an error. However, you need to reach out to ask him. -
Philippe Chantreau at 05:28 AM on 22 September 2011CERN - Saying Nothing About Cosmic Ray Effects on Climate
Tblakeslee you're making extraordinary claims. These require extraordinary evidence. You're also dismissing the work, dedication and accumulated knowledge of thousands of experts. You're saying that someone who was not a physicist understood the Sun better than solar physicists. But you have no real evidence of all this and no peer-reviewed science to support it. You can not just say stuff is true because you feel like it should be. The name for that is wishful thinking. There is no credible scientific evidence at this time that cosmic rays modulate cloud cover. There is not even a physical mechanism for ionized particles to grow to the size at which they can act as CCN. If you know of one, post a link. The correlation found by Svensmark between Forbush events and cloud cover changes is spurious at best. It's not only Lockwood but other teams that have looked at that supposed correlation and found it all but lacking. As Muoncounter pointed, Forbush events of any notable magnitude happen a few times a year. As I said earlier, the resulting cloud events must be massive in order to modulate climate. You eluded that question before, I'm asking again: Where is the evidence of that happening? Massive events are noticeable. A link would be appreciated. Sicentists don't understand the magnetic nature of the Sun eh? Right, that's why they thought it would be good to have the MDI imager on SOHO. I'm sorry but your demeaning remarks on solar scientists while you show limited knowledge and understanding yourself is rather damaging to your credibility. You should first prove (for real, in an objective way) that you understand things better before equating them to idiots (which is in essence what you did). The 11 years cycle, wich is the only true cyclic variation identified so far in the Sun is known exactly as a magnetic phenomenon by the way. ENSO does not show any true cyclical behavior. It is a quasiperiodic oscillation. What scientific work (not blog posts) can you refer to that links a fairly well established 11 year cycle with a quasiperiodic oscillation? One would have to go fetch some exotic harmonics to establish a link, I guess it might be feasible if you try hard enough. For now, I'll go by NOAA's take: "External forcing from volcanic eruptions (submarine or terrestial) have no connnection with El Niño. Nor do sunspots as far as we know." You've said how you "feel". Reality does not care one bit how we feel, how about bringing some substance? -
pielkesr at 05:26 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Hyperactive Hydrologist - re #66 As we write in Matsui, T., and R.A. Pielke Sr., 2006: Measurement-based estimation of the spatial gradient of aerosol radiative forcing. Geophys. Res. Letts., 33, L11813, doi:10.1029/2006GL025974. http://pielkeclimatesci.files.wordpress.com/2009/10/r-312.pdf 'This paper evaluates the mean and spatial gradient of aerosol radiative forcing in comparison with that of the well-mixed GHG. The appropriate metric to assess the importance of the gradient of diabatic heating is the resulting gradients in the horizontal pressure field that fundamentally drives the atmospheric circulation [Gill, 1982]." Diabatic heating from land use/land cover change, if of a similar magnitude as with aerososl, would alter atmospheric circulations. These include the PDO, ENSO, NAO etc which, in my view, are hemispheric atmospheric-ocean circulation features. This is what land use/land cover change could also have a larger scale effect on the climate system than the very well documented regional effects where land use/land cover has occurred.Moderator Response: [John Hartz] Pleass define tha acronyms that you have used and the term, "diabatic heating." -
Albatross at 05:23 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Dr. Pielke @94, "3) Climate models are certainly not perfect, but are useful tools which have made fairly accurate climate projections thus far. No, they models have not shown skill at predicting changes in regional climate statistics." With respect, you are shifting the goal posts and arguing strawmen. Dana was very likely referring to 'global climate', and you insist on focusing on regional impacts. Yes, those are of course important, and you seem to be dismissing out of hand the wealth of work that has been done on downscaling. Additionally, you know as well as I do that because NWP models (e.g., WRF) accurately or perfectly simulating certain mesoscale features/processes does not render them useless or of no value. Besides, we know that despite their imperfections and limitations NWP models are very useful, as are AOGCMs; you also ignore the fact that they continue to improve. I find it odd that AOGCMs are good enough to support your view that regional and local land use change is a first-order global climate driver (e.g., you citing Takata et al.) and by extension that we should place less focus on Carbon, but you then turn around and say that those same models are no use for guiding climate policy. Regardless, paleo climate shows us that we best take the most prudent path and significantly reduce our emissions to avoid some major changes. Models are but one of several tools that we can use for guidance. "I also have concluded that the computationally expensive climate models, when used for multi-decadal predictions, have not told us anything of demonstrated added value beyond what can be achieved with just global energy balance models." I respectfully disagree, and so do many of your colleagues. I also note that this is a logical fallacy on your part. You are at the same time arguing that simple models are good at today's sophisticated AOGCMs while also arguing that the models are not complex enough and do not account adequately for land cover and land use changes or adequately simulate the surface processes. You seem to want to have it both ways. And no, simple energy balance models do not cut it, just as Roy's simple one-box model does not cut it (see Trenberth et al. 2011). Those type of models are useful for teaching students the basic concepts and for conducting simple experiments.Moderator Response: [John Hartz] Please add a key defining the acronyms that you have used in this post. -
pielkesr at 05:18 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Tom Curtis re: #76 see http://pielkeclimatesci.wordpress.com/2010/12/01/the-role-of-soot-in-the-climate-system-an-excellent-article-in-the-economist/ and other links on soot that I included there. Soot appears to be a major factor also in the Arctic sea ice. In terms of glaciers in the Himalayas, several years ago Lonnie Thompson showed me ice core data from several glaciers there. The appearance of soot in recent years was obvious, and I remarked to him than that this needs to be factored in in terms of melt. -
pielkesr at 05:14 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Rob Honeycutt - Thanks for your comments. When I decided to engage with Skeptical Science, it was to reach out for a dialog. There is too much "tribalism" in weblogs, and they often wind up so partisan that only those in conformity with the views of the weblog host so there. I hope I encourage others on all sides to contribute and debate on all of the climate weblogs. I am disappointed to see the snide (snarky) comments from some and hope they realize that a positive interaction, even when we disagree, is not only more pleasant but also permits the development of consensus on at least some of the issues. -
pielkesr at 05:08 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Albatross - You write 'Dr. Pielke asserts quite confidently here and elsewhere that land use and land cover change are first-order global climate driver. As I mentioned before, he is reluctant to place a figure on that in terms of global radiative forcing." Lets assume it is zero as regions of positive and negative radiative forcing average out. Land use/land cover change, however, is still a first order climate forcing as it can alter regional climate more than that caused by the radiative forcing of added CO2. Also the alterations in spatial diabatic heating from this heterogenous forcing may alter large scale circulation features such as Asian monsoon, the NAO etc. I do recommend you read our paper..... Also, you write "Regardless, nowhere in your book, that I can see, (even in Chapter 12 "Model Evaluation") do you entertain the notion that models are "hypotheses".' you will see this in the 3rd Edition which is due to the publisher later this Fall. Finally, on EOS, since I have actually reviewed Forum articles, I know they are reviewed. A paper does not have to be an original contribution to be reviewed. -
muoncounter at 05:06 AM on 22 September 2011CERN - Saying Nothing About Cosmic Ray Effects on Climate
tblakeslee#80: "clearly ignored the electrical plasma and magnetic effects " Really? So the satellites (ACE, SOHO, Orion and so on) continuously monitoring solar electrical and magnetic fields are ignored? The impact of solar mag variations and storms on communications, military hardware and spaceflight isn't important enough to be studied in detail? Here is a good, if somewhat dated review of what we've 'completely ignored'. "distrust of Svensmark is probably based on the attack on his work by Lockwood" No, my skepticism of Svensmark is based on the fact that no physical mechanism is proven to support his hypothesis and there is considerable evidence to the contrary. I am skeptical because this question is not settled; many self-proclaimed 'skeptics' turn a blind eye to that situation when it challenges their own beliefs. That phenomenon has been called 'one-sided skepticism.' "Cloud formation over oceans is where the cosmic rays have the strongest effect because over land there are plenty of dust particles to aid in cloud formation. " You seem to be unaware that dust moves from over land to over oceans quite freely and aerosols with impunity. Google 'Asian brown cloud' or have a look at this image (from earthobservatory) taken off the coast of Africa. As far as cosmic ray induced ionization leading to cloud formation, the effect is not substantiated any better or worse over ocean or land. Cosmic rays don't seem to care where they land.
As far as cosmic ray induced ionization leading to cloud formation, the effect is not substantiated any better or worse over ocean or land. Cosmic rays don't seem to care where they land.
-
Albatross at 05:04 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Dr. Piellke @ 93, You did not responded properly (to at least clearly) to John's question-- we do not really care whose idea it might have been (also the idea of moist-static energy and its role in say convection, has been around for a long time). John asked: "Do you agree with the findings of the paper summarized below?" I am also rather perplexed that you insist on keep touting Klotzbach et al. (2009) when that paper is known to have issues (some would argue serious issues). That fact was pointed out in the second post of this thread, but I'll post links here. Link 1, Link 2. Dr. Annan concludes: "Funny how this inconvenient result is now relegated to a "time period and location examined" when it was previously hypothesised to be representative of the global picture. There were no fewer than three other places where L07 was originally cited as being consistent with the K09/PM05 hypothesis, but it would obviously have been too painful for them to mention that their own observations of boundary layer lapse rates contradict their theory. Therefore, these statements have just been deleted." And I speak for my (and your) colleagues too when I say that it is considered poor form and inappropriate to repeatedly quote/cite one's own work as you seem to have a propensity to do. The reason being is that it limits the scope of the discussion and subject at hand, suggests the person is not open to alternative ideas beyond their own and also indicates that they are reluctant to consider the body of scientific evidence, and for these reasons it does not facilitate the advancement of science. -
pielkesr at 05:01 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
dana1981 See my answers to your questions below. Dr. Pielke, we would like to do a post summarizing where we agree and disagree. Do you concur or have any comments on the following summary of agreements? We Agree on: 1) The need to reduce human GHG emissions As I wrote in my earlier answer "I am very much in favor of energy sources which minimize the input off gases and aerosols into the atmosphere. Much of my career has been involved with reducing air pollution (both in research and in policy). What we should move towards is an economy with as small a footprint on the natural environment as possible." 2) That while CO2 is the largest single contributor to global warming, there are other factors which we must also address No; I am not convinced that CO2 is the largest annual global averaged positive radiative forcing [and I am interpreting that you mean human climate forcings and the global annual average). Sott, and a variety of other aerosols have quite substantial positive radiative forcings in the atmosphere, and for soot,at the surface on snow and ice also. These other positive radiative forcings arr discussed in some depth in National Research Council, 2005: Radiative forcing of climate change: Expanding the concept and addressing uncertainties. Committee on Radiative Forcing Effects on Climate Change. The National Academies Press, Washington, D.C., 208 pp. http://www.nap.edu/openbook/0309095069/html/ 3) Climate models are certainly not perfect, but are useful tools which have made fairly accurate climate projections thus far. No, they models have not shown skill at predicting changes in regional climate statistics. They are not ready to be used for accurate regional impact assessments as we discuss in Pielke Sr., R.A., R. Wilby, D. Niyogi, F. Hossain, K. Dairuku, J. Adegoke, G. Kallos, T. Seastedt, and K. Suding, 2011: Dealing with complexity and extreme events using a bottom-up, resource-based vulnerability perspective. AGU Monograph on Complexity and Extreme Events in Geosciences, in press. http://pielkeclimatesci.files.wordpress.com/2011/05/r-365.pdf In terms of significant failings in the global climate models, as just two examples, see Stephens, G. L., T. L’Ecuyer, R. Forbes, A. Gettlemen, J.‐C. Golaz, A. Bodas‐Salcedo, K. Suzuki, P. Gabriel, and J. Haynes (2010), Dreary state of precipitation in global models, J. Geophys. Res., 115, D24211, doi:10.1029/2010JD014532. http://europa.agu.org/?view=article&uri=/journals/jd/jd1024/2010JD014532/2010JD014532.xml&t=jd,2010,stephens Insightful Interview In EOS Of Dr. De-Zheng Sun “Climate Dynamics: Why Does Climate Vary?” http://pielkeclimatesci.wordpress.com/2011/09/02/insightful-interview-in-eos-of-dr-de-zheng-sun-climate-dynamics-why-does-climate-vary/ I also have concluded that the computationally expensive climate models, when used for multi-decadal predictions, have not told us anything of demonstrated added value beyond what can be achieved with just global energy balance models. By so closely linking policy to these models, we are doing more harm than good in developing effective climate and energy policies. -
pielkesr at 04:47 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
John Hartz - Peterson et al based there analysis on our proposal to calculate surface moist enthalpy; see, for example, Pielke Sr., R.A., C. Davey, and J. Morgan, 2004: Assessing "global warming" with surface heat content. Eos, 85, No. 21, 210-211. http://pielkeclimatesci.wordpress.com/files/2009/10/r-290.pdf Davey, C.A., R.A. Pielke Sr., and K.P. Gallo, 2006: Differences between near-surface equivalent temperature and temperature trends for the eastern United States - Equivalent temperature as an alternative measure of heat content. Global and Planetary Change, 54, 19–32. http://pielkeclimatesci.wordpress.com/files/2009/10/r-268.pdf Fall, S., N. Diffenbaugh, D. Niyogi, R.A. Pielke Sr., and G. Rochon, 2010: Temperature and equivalent temperature over the United States (1979 – 2005). Int. J. Climatol., DOI: 10.1002/joc.2094.http://pielkeclimatesci.wordpress.com/files/2010/02/r-346.pdf In the last paper, we also found that "Even though most of the magnitude of TE is explained by T , the moisture component nduces larger trends and variability of TE relative to T." [note" TE is the measure of the moist enthalpy and T is the dry bulb temperature]. There remains the issue, however, as to how spatially representative are the surface land observations used in their study (and ours). Unless similar amount of water (per kg) went into the lower troposphere, however, the difference between surface air and lwer tropospheric heat content trends will be even larger than we found for the dry bulb temperatures; see Klotzbach, P.J., R.A. Pielke Sr., R.A. Pielke Jr., J.R. Christy, and R.T. McNider, 2009: An alternative explanation for differential temperature trends at the surface and in the lower troposphere. J. Geophys. Res., 114, D21102, doi:10.1029/2009JD011841. http://pielkeclimatesci.wordpress.com/files/2009/11/r-345.pdf Klotzbach, P.J., R.A. Pielke Sr., R.A. Pielke Jr., J.R. Christy, and R.T. McNider, 2010: Correction to: "An alternative explanation for differential temperature trends at the surface and in the lower troposphere. J. Geophys. Res., 114, D21102, doi:10.1029/2009JD011841", J. Geophys. Res., 115, D1, doi:10.1029/2009JD013655. http://pielkeclimatesci.wordpress.com/files/2010/03/r-345a.pdf -
Dave123 at 04:06 AM on 22 September 2011SkS Responses to Pielke Sr. Questions
Moderator: Is there a place where I can take MPaul to task for the no-such thing as any settled science Meme? As someone who IS a working scientist, albeit not a climate scientist, I have great objections to non-scientists making such context-free statements. I run into this all the time in the real world...it reflects, if mpaul is American the long-running anti-intellectual streak that equates one person's willing ignorance with another perons's years of experimental research. Gravity is settled. The germ-theory of disease is settled. DNA as the stuff of heredity is settled. And given mpaul's last statement, I think he's a AGW proponent unable to confront the fact that he's in denial about it. Presumably there are elements that stick in his craw, but the weight of evidence is smothering him.Moderator Response: [muoncounter] How about The science isn't settled? -
Bob Lacatena at 04:05 AM on 22 September 2011CERN - Saying Nothing About Cosmic Ray Effects on Climate
80, tblakeslee,Cosmic ray assisted clouds over the ocean modulate the amount of solar heating of the ocean but with a large flywheel effect.
I find it odd that you can so definitively make such a declaration with no evidence whatsoever, and you in another breath have said:The physicists are far from understanding the sun.
Citations please. Support your assertions with hard evidence. -
tblakeslee at 03:49 AM on 22 September 2011CERN - Saying Nothing About Cosmic Ray Effects on Climate
Landscheidt was vague because he is not a physicist and was mainly observing interactions driven by planetary movement that had a profound effect on the sun through gravity. The physicists are far from understanding the sun. They have clearly ignored the electrical plasma and magnetic effects which are important here. Here is a link to a minority view which I feel is more on track with the true electric/magnetic nature of the sun: http://www.electric-cosmos.org/sun.htm Your distrust of Svensmark is probably based on the attack on his work by Lockwood in 2007. This attack was based on many errors which are covered well in this rebuttal: http://members.shaw.ca/sch25/FOS/Climate_Change_Science.html#Hockey Lockwood tried incorrectly to smooth the 11-year solar cycle and thus destroyed most of the data. In reality, the la nina and el nino cycles result from solar cycles and profoundly affect temperatures. They must not be averaged out. Cloud formation over oceans is where the cosmic rays have the strongest effect because over land there are plenty of dust particles to aid in cloud formation. Most of the world is ocean. Cosmic ray assisted clouds over the ocean modulate the amount of solar heating of the ocean but with a large flywheel effect.
Prev 1503 1504 1505 1506 1507 1508 1509 1510 1511 1512 1513 1514 1515 1516 1517 1518 Next































 Arguments
Arguments



























