Frequently Asked Questions About Ocean Acidification
Posted on 4 January 2013 by Rob Painting
Skeptical Science is rather fortunate to have had three experts on ocean chemistry write an entire series (OA not OK) about ocean acidification (OA). Now the Woods Hole Oceanographic Institute (WHOI) has released a FAQ document, prepared by some of the world's foremost ocean acidification researchers, which answers many of the frequently asked questions about OA.
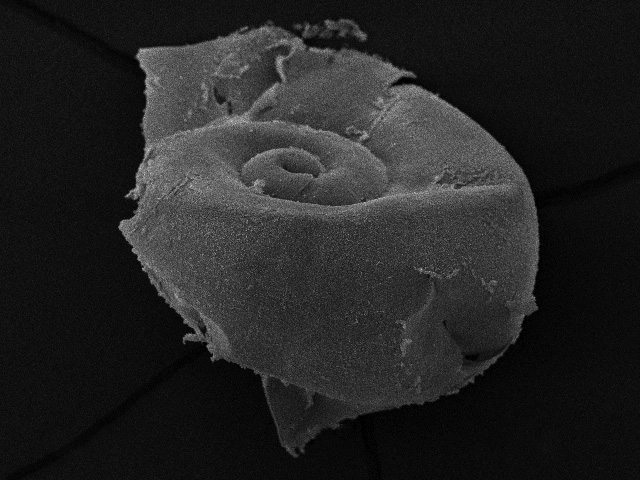
Figure 1 - A scanning electron microsope image of the pteropod (marine snail) Limacina helicina antarctica showing acute levels of shell dissolution. This species is a cornerstone of the Antarctic marine food web and is at imminent risk due to ocean acidification. Despite possessing a highly corroded shell, the pictured specimen was alive at point of capture. Image courtesy of the British Antarctic Survey.
As the above image graphically demonstrates, ocean acidification is not some far-flung future problem, it is a dire threat to many marine organisms that is happening in our oceans right now, and will progressively get worse with continued fossil fuel emissions.
So if you happen to be traversing the blogosphere and stumble upon some contrarian spouting pseudo-scientific gibberish about ocean acidification, not only do you have the SkS OA not OK series of blog posts with which to educate lurkers, but the WHOI FAQ document is also likely to provide a factual response to the typical climate contrarian talking points.
Climate-related myths tend to propagate like rabbits in the absence of scientific facts, so please disseminate the WHOI FAQ as widely as possible.































 Arguments
Arguments























 0
0  0
0 Although the rate of PETM ocean acidification was much smaller than present-day it still resulted in the extinction of some species.
Although the rate of PETM ocean acidification was much smaller than present-day it still resulted in the extinction of some species.







Comments