 Arguments
Arguments
 Software
Software
 Resources
Comments
Resources
Comments
 The Consensus Project
The Consensus Project
 Translations
Translations
 About
Support
About
Support


Latest Posts
- Skeptical Science New Research for Week #10 2026
- Will climate change bring more major hurricane landfalls to the U.S.?
- Just have a Think - The Primary Energy Fallacy finally laid to rest!
- The AI-Augmented Scientist
- 2026 SkS Weekly Climate Change & Global Warming News Roundup #09
- Skeptical Science New Research for Week #9 2026
- Fossil fuel pollution’s effect on oceans comes with huge costs
- Fact brief - Do solar panels work in cold or cloudy climates?
- After a major blow to U.S. climate regulations, what comes next?
- 2026 SkS Weekly Climate Change & Global Warming News Roundup #08
- Skeptical Science New Research for Week #8 2026
- Introducing the Climate Brink Dashboard
- Climate Adam - Climate Scientist Reacts to AI Overlords
- Trump just torched the basis for federal climate regulations. Here’s what it means.
- 2026 SkS Weekly Climate Change & Global Warming News Roundup #07
- Skeptical Science New Research for Week #7 2026
- These key strategies could help Americans get rid of their cars
- Fact brief - Can nearby solar farms reduce property values?
- Sea otters are California’s climate heroes
- 2026 SkS Weekly Climate Change & Global Warming News Roundup #06
- Skeptical Science New Research for Week #6 2026
- The future of NCAR remains highly uncertain
- Fact brief - Can solar projects improve biodiversity?
- How the polar vortex and warm ocean intensified a major US winter storm
- 2026 SkS Weekly Climate Change & Global Warming News Roundup #05
- Help needed to get translations prepared for our website relaunch!
- Skeptical Science New Research for Week #5 2026
- Climate Variability Emerges as Both Risk and Opportunity for the Global Energy Transition
- Fact brief - Are solar projects hurting farmers and rural communities?
- Winter 2025-26 (finally) hits the U.S. with a vengeance
Archived Rebuttal
This is the archived Basic rebuttal to the climate myth "Ocean acidification isn't serious". Click here to view the latest rebuttal.
What the science says...
|
Ocean acidification threatens entire marine food chains. |
At-a-glance
Have you heard of ocean acidification? Does it mean that if you go swimming in the sea, you are liable to dissolve? No. You'll be OK because you are not a calcifying organism, such as a mollusc, a coral or a sea-urchin.
So why is ocean acidification serious? Because it can potentially lead to massive collapse of marine food-chains. Let's take a look at what the term means.
The pH scale, which measures acidity and alkalinity of water-based chemical solutions, runs from 0 (highly acidic) to 14 (highly alkaline), with pH 7 being the neutral halfway point. Importantly, the scale is logarithmic, meaning that a jump of one point towards zero means a tenfold increase in acidity.
Acidification simply means lowering the pH value from any point on the pH scale towards zero. It's similar to the way we talk about temperatures. If the pH of a solution shifts from 9 to 8, that is acidification, even though the pH is still on the alkaline side of neutral. Likewise, if the temperature rises from -40°C to -15°C, it has noticeably warmed, even though it's still darned cold.
Now, typical seawater is slightly alkaline at around pH 8.1. Rainwater, which always contains dissolved carbon dioxide (the old name for which was 'carbonic acid gas'), has a more acidic pH of around 5.6. You have likely visited or watched footage of spectacular caves, have you not? All carved out by carbonic acid, dissolving solid limestone over many thousands of years.
Carbonic acid is not only present dissolved in raindrops. It also forms by the dissolving of carbon dioxide at the air-water interface of our oceans. The more carbon dioxide in the air, the more goes into the oceans, driving their pH from 8.1 downwards. Now, the huge problem this creates, well before we get anywhere near the neutral value, is as follows.
Many marine organisms build and maintain their protective shells or skeletons from 'biogenic' calcium carbonate. The word biogenic means made by living things. These creatures extract the calcium and carbonate ions dissolved in seawater and combine them together. Under normal conditions, such calcium carbonate is stable in shallow waters. That's because dissolved carbonate ions are present in such high concentrations that the waters are said to be saturated with them.
But if seawater pH falls, even by a small amount, the concentration of dissolved carbonate ions falls. When that happens, biogenic calcium carbonate becomes more soluble and can start to dissolve. Depletion in dissolved carbonate ions thus makes it harder for such organisms to maintain their protective or skeletal structures. In the worst case scenario, the rate of calcium carbonate dissolution is faster than its formation. When that happens, mass-mortality of calcifying organisms can occur.
We're talking about critters that underpin entire marine food-chains here. Things from near-microscopic calcifying plankton to shellfish, lobsters and crabs the seafood we eat in other words. That's why ocean acidification is deadly serious.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
Not all of the CO2 emitted by human industrial activities remains in the atmosphere. Between 25% and 50% of these emissions over the industrial period have been absorbed by the world’s oceans, preventing atmospheric CO2 buildup from being much, much worse. But this atmospheric benefit comes at a cost.
As ocean waters absorb CO2 they become more acidic. This does not mean the oceans will become like the acids one encounters in a chemistry lab. However, marine life can be highly sensitive to slight changes in pH levels and any drop in pH is an increase in acidity, even in an alkaline environment. Worse, the pH scale is logarithmic, meaning that for each single-digit decline in pH, acidity (defined as hydrogen ion activity) rises tenfold.
Surface seawater pH has been relatively stable over recent geological time, fluctuating between cold glacial periods (pH 8.3) and warmer interglacials (pH 8.2). But since the Industrial Revolution, average seawater pH has dropped towards a recent figure of less than 8.06, an approximately 30% increase in acidity (fig. 1). This is a faster change than any over the past 50 million years (Rhein et al, 2013, available from IPCC here).
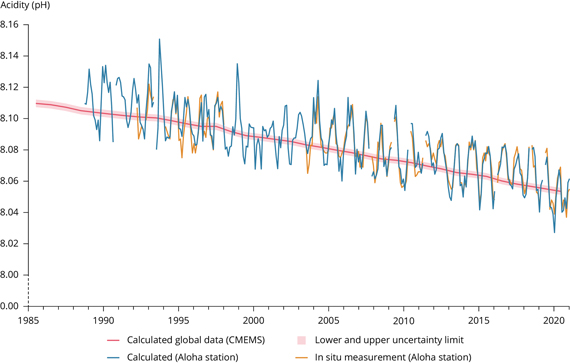
Fig. 1: Decline in ocean pH measured at the Aloha station (in the Pacific Ocean off Hawaii) and yearly mean surface seawater pH reported on a global scale Source: European Environment Agency (Copernicus Marine Service).
Because of its inextricable link with CO2 emissions, this rate of acidification is projected to accelerate even further through the 21st century under a business-as-usual scenario with potentially catastrophic impacts to marine ecosystems (Bindoff et al. 2019 (PDF from IPCC)). These trends are becoming clearer globally.
According to the IPCC's Sixth Assessment Report (AR6), there is, " a very likely rate of decrease in pH in the ocean surface layer of 0.016 to 0.020 per decade in the subtropics and 0.002 to 0.026 per decade in subpolar and polar zones since the 1980s. Ocean acidification has spread deeper in the ocean, surpassing 2000 m depth in the northern North Atlantic and in the Southern Ocean (fig. 2)."
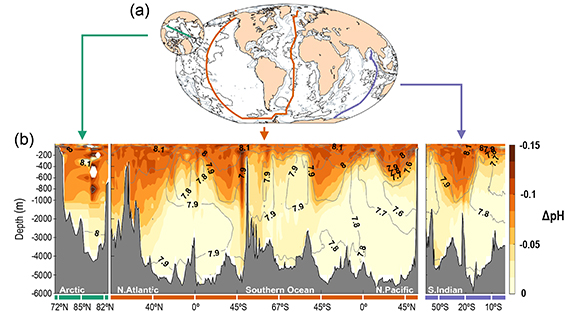
Fig. 2: Spread of ocean acidification from the surface into the depths since pre-industrial times. (a) Map showing the three transects used to create the cross sections shown in (b), showing the vertical sections of the changes in pH between 1800–2002 due to anthropogenic CO2 emissions; the darker the colours the greater the change. Contour lines are their contemporary values in 2002. Graphic sourced from IPCC AR6. (Lauvset et al. 2020).
Such changes in ocean chemistry, if allowed to occur, will be irreversible for many thousands of years. The biological consequences could last much longer.
How do we know that? Through the geological record. When mass-extinctions have occurred, most of them are tied-into unimaginably severe episodes of volcanism, at a scale never witnessed by humans. But the carbon footprint of such cataclysms has in fact been similar to our own. And what do we see as a consequence of such events? The fossil record shrinks in terms of its biodiversity and there are what we call 'reef-gaps', periods of several million years during which coral reefs - large highly diverse colonies of corals and myriad other species - were to all intents and purposes absent.
The reason why reef-gaps occur at such times is because as surface waters become more acidic, it becomes more difficult for corals, shellfish and other calcifying organisms to form and maintain the hard calcium carbonate skeletons or shells necessary for their survival. When things start getting really bad, that calcium carbonate dissolves away as fast as it can be deposited - that means curtains for such critters.

Fig. 3: just some of the life-forms at deadly risk from the acidification of near-surface ocean waters.
Coral reefs provide a home for more than 25% of all oceanic species, so you can see why this matters so much. Some calcifying organisms, such as the tiny pteropods (fig. 3), underpin many oceanic food chains: take them out of the system and down those food-chains come crashing. Many communities around the world, constituting millions of people, are at the apex of such food-chains, relying on seafood as part of a healthy diet. You should now be able to see the problem. Like a thief in the night, ocean acidification is creeping up on us, while we sleep on in blissful unawareness.
Updated on 2023-06-25 by John Mason.
THE ESCALATOR

(free to republish)
























































