How do we know more CO2 is causing warming?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
An enhanced greenhouse effect from CO2 has been confirmed by multiple lines of empirical evidence. |
|||||||
Climate Myth...
Increasing CO2 has little to no effect
"While major green house gas H2O substantially warms the Earth, minor green house gases such as CO2 have little effect.... The 6-fold increase in hydrocarbon use since 1940 has had no noticeable effect on atmospheric temperature ... " (Environmental Effects of Increased Atmospheric Carbon Dioxide)
At-a-glance
To make a statement like, "minor greenhouse gases such as CO2 have little effect", is to ignore 160 years of science history. So let's look at who figured out the heat-trapping properties of carbon dioxide and when.
Experiments involving various gas mixtures had demonstrated the heat-trapping properties of water vapour, CO2 and methane in the 1850s. But those effects were yet to be quantified - there were no meaningful numbers. It was to be another 40 years before that happened.
Swedish scientist Svante Arrhenius (1859-1927) was the person who crunched the numbers. The results were presented in a remarkable paper, "On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground", in 1896.
The many calculations in the 1896 paper include estimates of the amounts of CO2 increase or decrease required to drive the climate into a different state. One example used was the Hothouse climate of the Cenozoic, around 50 million years ago. Another was the glaciations of the last few hundred millennia.
To get a temperature rise of 8-9°C in the Arctic, Arrhenius calculated that CO2 levels would have to increase by 2.5 to 3 times 1890s levels. To lower the temperature 4–5°C to return to glacial conditions, he calculated a drop in CO2 was needed of 0.62-0.55 times 1890s levels.
We know CO2 levels in the 1890s from ice-core data. They were around 295 ppm. Let's do the sums. A reduction factor of 0.55 to 0.62 on 295 ppm gives 162.2-183.9 ppm. Modern ice-core measurements representing the past 800,000 years show that in glacial periods, CO2 levels fell to 170-180 ppm.
What we now know due to additional research since 1896 when Arrhenius worked on this, is that CO2 was an essential 'amplifying feedback'. That means changes triggered by long term, cyclic variations in Earth's orbit cause warming or cooling and CO2 release or entrapment in turn. Those changes in CO2 levels affected the strength of Earth's greenhouse effect. Changes in the strength of the greenhouse effect then completed the job of pushing conditions from interglacial to glacial - or vice-versa.
Arrhenius also made an important point regarding water vapour: "From observations made during balloon voyages, we know also that the distribution of the aqueous vapour may be very irregular, and different from the ideal mean distribution." This statement holds true today: water vapour is a greenhouse gas but because water exists in gas, liquid and solid forms in the atmosphere, it is continually cycling in and out of the air. It is distributed in a highly uneven fashion and is uncommon in the upper atmosphere. That's where it differs from CO2.
Once CO2 is up there, it's up there for a long time. As a consequence it has a pretty even distribution: 'well-mixed' is the term. As Arrhenius quantified all that time ago, once it's up there it constantly absorbs and re-radiates heat in all directions. That's why dumping 44 billion tons of it into our atmosphere in just one year (2019 - IPCC Sixth Assessment Report 2022) is a really bad idea.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
Good scientific theories are said to have ‘predictive power’. In other words, armed only with a theory, we should be able to make predictions about a subject. If the theory’s any good, the predictions will come true.
Here’s an example: when the Periodic Table of the chemical elements was proposed in 1869, many elements were yet to be discovered. Using the theory behind the Periodic Table, the Russian chemist Dmitri Mendeleev was able to predict the properties of germanium, gallium and scandium prior to their discovery in 1886, 1875 and 1879 respectively. His predictions were found to be correct.
The effect on Earth's greenhouse effect of adding man-made CO2 is predicted in the theory of greenhouse gases. This theory was first proposed by Swedish scientist Svante Arrhenius in 1896, based on earlier work by Fourier, Foote and Tyndall. Many scientists have refined the theory since Arrhenius published his work in 1896. Nearly all have reached the same conclusion: if we increase the amount of greenhouse gases in the atmosphere, the Earth will warm up.
Where there is less agreement is with respect to the exact amount of warming. This issue is called 'climate sensitivity', the amount the temperatures will increase if CO2 is doubled from pre-industrial levels. Climate models have predicted the least temperature rise would be on average 1.65°C (2.97°F) , but upper estimates vary a lot, averaging 5.2°C (9.36°F). Current best estimates are for a rise of around 3°C (5.4°F), with a likely maximum of 4.5°C (8.1°F). A key reason for this range of outcomes is because of the large number of potential climate feedbacks and their variable interactions with one another. Put simply, some are much better understood than others.
What Goes Down…
The greenhouse effect works like this: Energy arrives from the sun in the form of visible light and ultraviolet radiation. The Earth then emits some of this energy as infrared radiation. Greenhouse gases in the atmosphere 'capture' some of this heat, then re-emit it in all directions - including back to the Earth's surface.
Through this process, CO2 and other greenhouse gases keep the Earth’s surface 33°Celsius (59.4°F) warmer than it would be without them. We have added 42% more CO2, and temperatures have gone up. There should be some evidence that links CO2 to the temperature rise.
So far, the average global temperature has gone up by more than 1 degrees C (1.9°F):
"According to an ongoing temperature analysis led by scientists at NASA’s Goddard Institute for Space Studies (GISS), the average global temperature on Earth has increased by at least 1.1° Celsius (1.9° Fahrenheit) since 1880. The majority of the warming has occurred since 1975, at a rate of roughly 0.15 to 0.20°C per decade."
The temperatures are going up, just like the theory predicted. But where’s the connection with CO2, or other greenhouse gases like methane, ozone or nitrous oxide?
The connection can be found in the spectrum of greenhouse radiation. Using high-resolution FTIR spectroscopy, we can measure the exact wavelengths of long-wave (infrared) radiation reaching the ground.
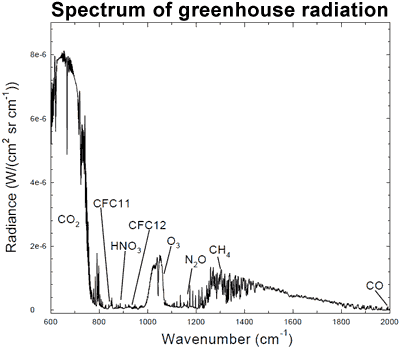
Figure 1: Spectrum of the greenhouse radiation measured at the surface. Greenhouse effect from water vapour is filtered out, showing the contributions of other greenhouse gases (Evans 2006).
Sure enough, we can see that CO2 is adding considerable warming, along with ozone (O3) and methane (CH4). This is called surface radiative forcing, and the measurements are part of the empirical evidence that CO2 is causing the warming.
...Must Go Up
How long has CO2 been contributing to increased warming? According to NASA, “Two-thirds of the warming has occurred since 1975”. Is there a reliable way to identify CO2’s influence on temperatures over that period?
There is: we can measure the wavelengths of long-wave radiation leaving the Earth (upward radiation). Satellites have recorded the Earth's outgoing radiation. We can examine the spectrum of upward long-wave radiation in 1970 and 1997 to see if there are changes.
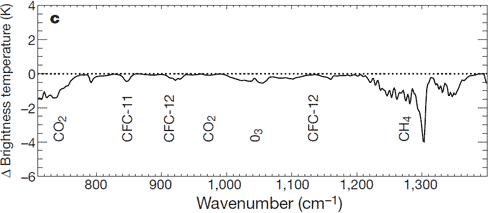
Figure 2: Change in spectrum from 1970 to 1996 due to trace gases. 'Brightness temperature' indicates equivalent blackbody temperature (Harries et al. 2001).
This time, we see that during the period when temperatures increased the most, emissions of upward radiation have decreased through radiative trapping at exactly the same wavenumbers as they increased for downward radiation. The same greenhouse gases are identified: CO2, methane, ozone and so on.
The Empirical Evidence
As temperatures started to rise, scientists became more and more interested in the cause. Many theories were proposed. All save one have fallen by the wayside, discarded for lack of evidence. One theory alone has stood the test of time, strengthened by experiments.
We have known CO2 absorbs and re-emits longwave radiation, since the days of Foote, Tyndall and Arrhenius in the 19th Century. The theory of greenhouse gases predicts that if we increase the proportion of greenhouse gases, more warming will occur.
Scientists have measured the influence of CO2 on both incoming solar energy and outgoing long-wave radiation. Less longwave radiation is escaping to space at the specific wavelengths of greenhouse gases. Increased longwave radiation is measured at the surface of the Earth at the same wavelengths.
Last updated on 16 July 2023 by John Mason. View Archives































 Arguments
Arguments






































I am interested in the climate debate and have noticed a pattern where the pro camp resort to insults and continue to claim its science where the deniers tend to look at the actual real time data regarding warming,sea level etc.You see it should have started happening by now.Curious? feel free to insult me
[DB] Ideology, fake-ad-hominem and off-topic snipped.
Yeah right, Duncan.
Please specify the actual real time data which the scientists ignore /misinterpret.
Yes, deniers do sometimes look at the data ~ they see it, but they do not observe it. [courtesy: Sherlock Holmes]
I looked at all the historic records for all the major cities in Canada and there has been no change.It should of started by now.I am keeping it simple and would have to go back to the source but all the predictions for as early as 2000 have not come true.The show I saw was filmed in a basement in Manhattan which according to the modelling should be under water but it is not.The data for the extreme change is all being fudged and you cant do that.I will be surprised if I am allowed to ask these questions and expect to get Moderated
[DB] Conspiracy-thinking, ideology and off-topic snipped.
I reread the question.NASA sea level data does not corelate to the actual tide data around the globe and its different by a lot.There is no relationship with alledged climate change and bushfires in Australia.I live here and it has happened as long as I can remember.Its all about reducing fuel load.We dont get fires in the middle of the country cos there is nothing to burn
[DB] Off-topic snipped.
Duncan, I ask again :-
Please specify the actual real time data which the scientists ignore /misinterpret.
I will pick sea levels,The actual tide reading at locations around the world are real and recordable but the NASA data is different and the modelling even wilder.Its hard to take it serious when predictions are made but do not come true in real time.I have access to claims that areas where I live would be underwater by 2020.Well can we agree its 2020 and the areas are still there and not flooded.I was wrong about the sea levels in Fremantle they have gone up 200mm but its over 160 years and the sea is lower at French Guyana.The magnetic poles are moving and undersea volcanoes occur plus localized silting.There is a claim here locally that the land is sinking because we are using a lot of groundwater for consumption.You can easy find all the data online or would you like me to post pictures with wriggly lines on it.I am keen to find the truth and need some actual proof.On another forum a poster showed before and after pictures of a glacier the first is from 1940 and its all iced up the second is recent and it shows all the ice gone except on the top of the mountain.The only problem I picked up on was the second photo is clearly much closer than the first and the water level is way down on the first photo where they were standing at the edge of the lake.The second photo they would have been 30 feet under and it is definitely the same place.That individual never posted again once I pointed this out.Makes it hard to take it for real.What do you have.Claims based on what should happen.Can anyone in the universe show me where its flooded and not Norfolk because that always floods on the spring tide.I have seen pictures of 1940 airbases on pacific Islands that are only 1 metre above sea level and they are still there
[DB] You are off-topic. There are thousands of posts here on virtually every subject pertaining to climate change science, each with it's own discussion thread. Use the Search tool to find the most appropriate one. If you persist in being off-topic, as you have been, your posting privileges will be rescinded. There will be no further Warnings on this matter.
Off-topic snipped.
Sorry Duncan, but you are still not giving any real evidence that the scientists are ignoring data.
Science is published in peer-reviewed papers in respected scientific journals. Not by Al Gore or Christopher Monckton or Tony Heller in shonky crap like Breitbart or NoTricksZone or WattsUpWithThat.
Reputable scientific journals, Duncan, where it gets examined and criticized by experts. The data can also be extensively discussed on reputable websites (such as this one).
If you are having problems in understanding the real factual state of things, Duncan, then it is likely because the real scientists know something that you don't know about climate. The scientists are not ignoring data. And so far, you have not demonstrated any data that they are (allegedly) ignoring.
Duncan, you are well off-topic for this thread, which concerns CO2 and Warming. If you can find some genuine examples of what you believe is ignored information /data, then please bring it to everyone's attention in the proper thread for that topic.
My question is about greenhouse radiation spectrum. Is the radiation spectrum different at surface of blue water ocean and surface of land?
Any spectrum curve I've seen that has location is over land. If there is a difference then the amount of energy available to be absorbed by CO2 would be significant in the discussion.
Whari @408 : Sorry, but I am confused by the wording of your question. Can you re-state the point you are discussing?
Am I right in assuming you are talking about the upward Infra-Red radiation spectrum detected by satellites? Or something else? The satellites can detect reflected radiation (visible and near-visible light) or they can detect IR radiation emitted from land / sea / clouds / atmospheric gas (H2O; CO2; CH4; etcetera).
From a GreenHouse point of view, the satellites are detecting upward IR radiation from the upper troposphere (so-called TOA - Top Of Atmosphere - which is at an altitude of approx 3 - 10 km, depending on which latitude and which of the GreenHouse Gasses you are considering). The upper atmosphere is a swirling mix of air (averaging of horizontal winds and vertical convections) and so is not directly connected to the ocean or land surface below.
Heat is lost upwards from ocean & land, by means of air convection & evaporation/re-condensation & Infra-Red radiation (from molecule to molecule in the air).
My apologies if I am misunderstanding you.
Whariwharangi - for all intents and purposes, the spectrum of the IR emitted is determined by surface temperature (Planck's law). Sea is generally cooler than land, but any modelling of emissions absolutely takes that into account.
I need some clarification about how greenhouse gases warm the atmosphere. I understand how the GHG warm up by absorbing certain infrared wavelengths. How does the other 99.9% warm? According to the National Weather Service, only 5 units of the heat comes from convective currents, presumably originating with conductance at the surface. https://www.weather.gov/jetstream/energy. Another 24 units comes from condensation of water vapor. 104 units comes from longwave radiation absorbed by GHG. How does the heat transfer from the small volume of GHG to the large volume of other gases? Is it conductance at the molecular level? Wouldn't this have to occur very rapidly, given the very high ratio of the two volumes? How does this affect the ability of the GHG to re-radiate in the wavelengths originally absorbed?
devcarr... Each IR photon leaving the surface is interacting with more than one molecule of CO2. Each photon is being absorbed and re-emitted many times. Some of the energy is transferred to other atmospheric molecules through collisions, but often it's being re-emitted only to collide with another GHG molecule. Some of that energy ends up back at the surface. Some eventually makes its way out to space.
Thanks, Rob Honeycutt..
Somehow the entire atmosphere is warming up, not just the GHG. A thermometer measures the nitrogen and oxygen just as much as the CO2 and water. You can't say the infrared radiation is warming the atmosphere if it only warms the GHG. I am asking how the heat transfers from the hot GHG to the cooler gases, which are 99.9% or more. I presume you are correct that some is transferred through collisions (conductance). I would like more details about how that happens, and how such a small fraction can heat up all the other gases so quickly.
devcarr @411,
The molecules of the atmosphere are in collision with each other at timescales measured in microseconds. The time for an excited CO2 molecule to use the excitement to emit an IR photon averages thousands of times longer. Thus the chances of an excited CO2 molecule being given time to emit an IR photon is very small. Effectively, this means pretty-much all the IR energy that bombards atmospheric gas and is absorbed by CO2 is transfered to the general atmospheric gas molecules as thermal energy.
But the large number of molecular collisions suffered by CO2, as well as robbing them of almost all incidents of them in an excited state before they can emit IR, also imparts this same excited state into many many more CO2 molecules. And it is this enlarged population of excited CO2 molecules that provides the CO2 molecules for a few of them with the chance to emit an IR photon.
This should make sense as it is the temperature of the gas, and thus the level of inter-molecular collisions, that determines the level of IR being emitted by the gas. And conversely, it is thus not the level of bombardment of IR photons that determines the level of IR being emitted from the gas.
devcarr: Pro scientist Eli Rabbett explained the details, and summarized "a quick estimate that only one out of 100,000 CO2 molecules excited into the (0,10,0) by collision or absorbing a photon, will emit." The vast majority either hang on to the energy or lose it by collision.
[TD] Fixed broken link.
Tom's link to "Eli Rabbet explained" seems to lead nowhere, but Eli has a full explanation on his blog:
http://rabett.blogspot.com/2013/04/this-is-where-eli-came-in.html
The short story is as MA Rodger explained.
Thanks Bob! (Apologies. I forgot to check my link after posting.)
I was just thinking more about this question coming from devcarr. It seems like a very common logical track that people take. How can one CO2 molecule possibly heat up 2500 other atmospheric molecules?
The mechanism of collisional transfer is fairly complex (per Eli's piece) for a non-scientist. Maybe the more simple answer is in regards to the relative number of IR photons and molecular collisions constantly taking place.
For one radiatively active greenhouse gas molecule to heat up 2500 other radiatively transparent gas molecules is quite easy, when you think of it in terms of the many millions (billions?) of IR photons hitting those GHG's every... second? millisecond? I'm not sure what the right number is.
People get stuck on the proportion of CO2 in atmosphere and forget the proportion of IR interacting with the CO2.
4% seems like a small no. but easy to forget that Avogadro's number is very large - there are a lot of molecules. Beside the sheer no. of photos per meter square per second, the other intuition to help guide understanding is how far on average will a photon travel on average before encountering a molecule of CO2. I suspect people that think 4% is a small no. will imagine that it easy for a photon to escape whereas the mean path length is more like a few meters.
Rob Honeycutt @418,
I don't think it is entirely correct to say the 0.041% CO2 warms up the rest of the atmosphere. It is the result of the GH-effect that provides the warming and that encompasses far more than the IR absorbed by CO2. As well as absorbing IR, the CO2 also emits IR. And as only GHGs do this, the prospect of an atmosphere with GHGs absent takes a bit of mental grappling.
But if there is CO2, within the 15 microns wave band.....
I would describe the CO2 as providing two things. First a fog that prevents the 15 micron wave band of IR from travelling very far, this by absorbing the IR. Imagine a candle in a thick fog. A few metres away and there is no sign of it. The thicker the CO2 fog the shorter the distance.
But there is the flip-side of this fog. The CO2 fog, as well as absorbing IR is also glowing, emitting IR. And because it is the fog itself that is glowing, the intensity of the glow is dependent on the temperature and not on the CO2 concentration. In the atmosphere, the lapse rate will thus give maximum glow close to the surface with the glow diminishing with altitude. The surface itself acts pretty-much as a mirror, reflecting back the 15 micron glow except being on average a little warmer than the surface air, it will glow a bit brighter.
The diminishing brightness with altitude will also mean a net flow of upward energy and when the quantity of CO2 above gets so thin to allow the IR to shoot off into space, the downward flux starts to faulter and that causes an evolution of the net upward energy flux, creating the one out into space which is dependent on the temperature of the atmosphere at that altitude emitting into space.
The effective size of a 0.041% CO2 concentration may also be worth a few words because, as Scaddenp @419 says, Avogadro's number is impressively large and that does make a bit of a nonsense of the “But it's only 0.041%!!” argument. I have (more correctly “had”) a couple of visualisations which hopefully shows how Avogadro's number makes a nonsense of such an argument and they might be worth setting out here for the thread.
MA Rogers... Thanks for that further clarification. One thing that I always wonder is, what would the temperature structure of the planet be without GHG's? Setting aside the fact that we'd have a snowball earth, what would a pure nitrogen-oxygen atmosphere do? There would certainly be some conduction from the surface and convection off of that. I have a hard time working out the results. I'm sure someone has modeled this. I've just never heard what the results were. Perhaps understanding this would help people to better understand why trace gases have such an important effect.
Rob Honeycutt @421,
I don't know of anybody attempting a model of Earthly climate without GHGs, with the exception of Lacis et al (2010) which modelled the cooling from present conditions up to the point when the ice-formation reached the ocean bed, that 50 years into the run. Their model still shows 10% of present atmospheric water vapour by that time.
Regarding a role for water vapour, my own thoughts turn to our recent ice ages and how a cold planet moves water poleward to form very large polar ice caps. These would be larger still without GHGs. And without interglacials to melt them, the ice caps would presumably become in-the-main part of geology. Outside the tropics I'd assume there would be nowhere with noon-day temperatures capable of forming liquid water. And if it entered the atmosphere from there, any water (or indeed sublimed ice) would tend to be carried poleward.
What the climate would be like with no GHGs, with atmospheric circulations no longer driven by high-altitude energy loss to space but solely by the larger surface temperature gradients? I think there are too many unknowns to speculate intelligently.
Here are the visualisations of 0.041% CO2 and the effect of molecules being so very small.
VISUALISATION 1
Although CO2 is only 0.041% of the atmosphere by volume, because air molecules are so very small, there is zero chance of a photon exiting from the surface into space without encountering a CO2 molecule; indeed many many CO2 molecules. This is simply because molecules are so small.
Imagine if this were not so. Imagine if molecules were 12km in diameter and the atmosphere as a result a sheet of air just one molecule thick. Crazy but imagine.
Only one part in 410 million of the world's surface would then have a giant CO2 molecule hanging over it. That is one part in 2,439. Thus from 99.959% of the planet, IR on a journey straight out to space would never encounter a CO2 molecule.
But molecules aren't that big. For one thing, they are not arrayed shoulder-to-shoulder but have a bit of room to whizz about, and spin and vibrate. To scale*, a CO2 molecule relative to the volume of atmosphere it occupies would be roughly 1km in diameter we gave it 12km of room to sit in. And if we now allow for the empty space round our CO2 molecules, the chances of free passage straight out into space is 187-times more likely*. So we can say for a single-molecule-sheet atmosphere, the likelihood of free passage is 99.9997807%. and the chances of hitting CO2 is just 1-in-456,000.
But molecules aren't 1km in diameter. So let's make them a bit smaller, say 1m in diameter. In a 12km-deep atmosphere, the chance of a free passage without hitting a CO2 molecule in one of the thousand layers of molecules sitting in their 12m square cubes above you is now 0.999997807^1000. So the chance of hitting CO2 is 1-in-3.
The chance of a free passage is becoming less certain for an IR photon.
But molecules aren't 1m big. And the smaller they get, the chances of a free passage shrink. With 1dm molecules, the chance of a free passage becomes less likely than not, 60-to-1 against. At 1cm, 645 quintillions-to-one against. The odds of free passage disappear as the size is reduced, at 1mm 1.25 x 10^187-to-1 against and quickly becoming so large that calculators cannot cope with such large numbers. And a CO2 molecule is actually 3 million times smaller than 1mm.
I shoulkd add that while it is true that not all CO2 molecules will be in a state to absorb an appropriate IR photon, the odds are so great, that is not very significant.
(*The usual number given for the size of a CO2 molecule is 0.33 nanometres diameter. From avogadro's number we can put the number of atmospheric molecules at 10^44 while our visualisation has an atmospheric volume of 510e12 x 12000 = 6e18 sq m. That would put each air molecule in a cube 4 nanometres wide. The box size is 12-times the diameter of a CO2 molecule. Within the projected box area of 16 sq nm, a CO2 molecule would project an area of 0.085 sq nm or one in 187 of the box projected area.)
VISUALISATION 2
There is today 3.2 trillion tons of CO2 in the atmosphere. The area of the earth is 510 million sq km or 510 trillion sq m so there is [3.2/510 =] 0.0063 tons CO2 above each square metre of the planet. The s.g. of dry ice is 1.7 so the volume of that 0.0063 tons CO2 in solid form with all the molecules stacked together is [0.0063/1.7 =] 0.0037 cu m. If you therefore spread this CO2 evenly over that square metre of planet Earth, you would get a sheet 3.7mm thick. And because there are [6e23 x 1 e6 / 44 =] 1.36e28 CO2 molecules per ton, the 3.7mm sheet will be 10.5 million molecules thick. (That puts the size of a CO2 molecule at 0.35nm.)
Of course, in the real atmosphere, the CO2 is spread out up into the stratosphere but an escaping IR photon on a straight-up journey will still have to negotiate 10.5 million CO2 molecules for a clean escape. In the real world, a very large proportion will not impede an individual IR photon so the path length of an IR photon is greatly underestimated by this visualisation. But it does demonstrate that the 0.041% concentration does not in any way prevent CO2 acting as a very powerful GHG.
What is the term for the layer near the top of the troposphere, where most infrared actually radiates away from the earth, because the air above has too few GHG to stop it? Is it just called the top of the troposphere? How much has that radiative layer gained in altitude since the start of the industrial revolution? As I understand it, that is the "extra blanket" that has forced warming of the earth.
If we stopped emitting CO2, methane, and nitrous oxide tomorrow, how much would the average surface temperature rise before we reached equilibrium?
[DB] Figure 3.9 (page 99) shows the persistence of elevated temperatures after emissions cease:
"regardless of when emissions cease, GMST remains approximately constant for the subsequent millennium"
That's based on:
Gillett et al 2011 - Ongoing climate change following a complete cessation of carbon dioxide emissions