The greenhouse effect and the 2nd law of thermodynamics
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
| |||
|
The 2nd law of thermodynamics is consistent with the greenhouse effect which is directly observed. |
|||||
Climate Myth...
2nd law of thermodynamics contradicts greenhouse theory
"The atmospheric greenhouse effect, an idea that many authors trace back to the traditional works of Fourier 1824, Tyndall 1861, and Arrhenius 1896, and which is still supported in global climatology, essentially describes a fictitious mechanism, in which a planetary atmosphere acts as a heat pump driven by an environment that is radiatively interacting with but radiatively equilibrated to the atmospheric system. According to the second law of thermodynamics such a planetary machine can never exist." (Gerhard Gerlich)
At a glance
Although this topic may have a highly technical feel to it, thermodynamics is a big part of all our everyday lives. So while you are reading, do remember that there are glossary entries available for all thinly underlined terms - just hover your mouse cursor over them for the entry to appear.
Thermodynamics is the branch of physics that describes how energy interacts within systems. That interaction determines, for example, how we stay cosy or freeze to death. You wear less clothing in very hot weather and layer-up or add extra blankets to your bed when it's cold because such things control how energy interacts with your own body and therefore your degree of comfort and, in extreme cases, safety.
The human body and its surroundings and energy transfer between them make up one such system with which we are all familiar. But let's go a lot bigger here and think about heat energy and its transfer between the Sun, Earth's land/ocean surfaces, the atmosphere and the cosmos.
Sunshine hits the top of our atmosphere and some of it makes it down to the surface, where it heats up the ground and the oceans alike. These in turn give off heat in the form of invisible but warming infra-red radiation. But you can see the effects of that radiation - think of the heat-shimmer you see over a tarmac road-surface on a hot sunny day.
A proportion of that radiation goes back up through the atmosphere and escapes to space. But another proportion of it is absorbed by greenhouse gas molecules, such as water vapour, carbon dioxide and methane. Heating up themselves, those molecules then re-emit that heat energy in all directions including downwards. Due to the greenhouse effect, the total loss of that outgoing radiation is avoided and the cooling of Earth's surface is thereby inhibited. Without that extra blanket, Earth's average temperature would be more than thirty degrees Celsius cooler than is currently the case.
That's all in accordance with the laws of Thermodynamics. The First Law of Thermodynamics states that the total energy of an isolated system is constant - while energy can be transformed from one form to another it can be neither created nor destroyed. The Second Law does not state that the only flow of energy is from hot to cold - but instead that the net sum of the energy flows will be from hot to cold. That qualifier term, 'net', is the important one here. The Earth alone is not a "closed system", but is part of a constant, net energy flow from the Sun, to Earth and back out to space. Greenhouse gases simply inhibit part of that net flow, by returning some of the outgoing energy back towards Earth's surface.
The myth that the greenhouse effect is contrary to the second law of thermodynamics is mostly based on a very long 2009 paper by two German scientists (not climate scientists), Gerlich and Tscheuschner (G&T). In its title, the paper claimed to take down the theory that heat being trapped by our atmosphere keeps us warm. That's a huge claim to make – akin to stating there is no gravity.
The G&T paper has been the subject of many detailed rebuttals over the years since its publication. That's because one thing that makes the scientific community sit up and take notice is when something making big claims is published but which is so blatantly incorrect. To fully deal with every mistake contained in the paper, this rebuttal would have to be thousands of words long. A shorter riposte, posted in a discussion on the topic at the Quora website, was as follows: “...I might add that if G&T were correct they used dozens of rambling pages to prove that blankets can’t keep you warm at night."
If the Second Law of Thermodynamics is true - something we can safely assume – then, “blankets can’t keep you warm at night”, must be false. And - as you'll know from your own experiences - that is of course the case!
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
Among the junk-science themes promoted by climate science deniers is the claim that the explanation for global warming contradicts the second law of thermodynamics. Does it? Of course not (Halpern et al. 2010), but let's explore. Firstly, we need to know how thermal energy transfer works with particular regard to Earth's atmosphere. Then, we need to know what the second law of thermodynamics is, and how it applies to global warming.
Thermal energy is transferred through systems in five main ways: conduction, convection, advection, latent heat and, last but not least, radiation. We'll take them one by one.
Conduction is important in some solids – think of how a cold metal spoon placed in a pot of boiling water can become too hot to touch. In many fluids and gases, conduction is much less important. There are a few exceptions, such as mercury, a metal whose melting point is so low it exists as a liquid above -38 degrees Celsius, making it a handy temperature-marker in thermometers. But air's thermal conductivity is so low we can more or less count it out from this discussion.
Convection

Figure 1: Severe thunderstorm developing over the Welsh countryside one evening in August 2020. This excellent example of convection had strong enough updraughts to produce hail up to 2.5 cm in diameter. (Source: John Mason)
Hot air rises – that's why hot air balloons work, because warm air is less dense than its colder surroundings, making the artificially heated air in the balloon more buoyant and thereby creating a convective current. The same principle applies in nature: convection is the upward transfer of heat in a fluid or a gas.
Convection is highly important in Earth's atmosphere and especially in its lower part, where most of our weather goes on. On a nice day, convection may be noticed as birds soar and spiral upwards on thermals, gaining height with the help of that rising warm air-current. On other days, mass-ascent of warm, moist air can result in any type of convective weather from showers to severe thunderstorms with their attendant hazards. In the most extreme examples like supercells, that convective ascent or updraught can reach speeds getting on for a hundred miles per hour. Such powerful convective currents can keep hailstones held high in the storm-cloud for long enough to grow to golfball size or larger.
Advection
Advection is the quasi-horizontal transport of a fluid or gas with its attendant properties. Here are a couple of examples. In the Northern Hemisphere, southerly winds bring mild to warm air from the tropics northwards. During the rapid transition from a cold spell to a warm southerly over Europe in early December 2022, the temperatures over parts of the UK leapt from around -10C to +14C in one weekend, due to warm air advection. Advection can also lead to certain specific phenomena such as sea-fogs – when warm air inland is transported over the surrounding cold seas, causing rapid condensation of water vapour near the air-sea interface.

Figure 2: Advection fog completely obscures Cardigan Bay, off the west coast of Wales, on an April afternoon in 2015, Air warmed over the land was advected seawards, where its moisture promptly condensed over the much colder sea surface.
Latent heat
Latent heat is the thermal energy released or absorbed during a substance's transition from solid to liquid, liquid to vapour or vice-versa. To fuse, or melt, a solid or to boil a liquid, it is necessary to add thermal energy to a system, whereas when a vapour condenses or a liquid freezes, energy is released. The amount of energy involved varies from one substance to another: to melt iron you need a furnace but with an ice cube you only need to leave it at room-temperature for a while. Such variations from one substance to another are expressed as specific latent heats of fusion or vapourisation, measured in amount of energy (KiloJoules) per kilogram. In the case of Earth's atmosphere, the only substance of major importance with regard to latent heat is water, because at the range of temperatures present, it's the only component that is both abundant and constantly transitioning between solid, liquid and vapour phases.
Radiation
Radiation is the transfer of energy as electromagnetic rays, emitted by any heated surface. Electromagnetic radiation runs from long-wave - radio waves, microwaves, infra-red (IR), through the visible-light spectrum, down to short-wave – ultra-violet (UV), x-rays and gamma-rays. Although you cannot see IR radiation, you can feel it warming you when you sit by a fire. Indeed, the visible part of the spectrum used to be called “luminous heat” and the invisible IR radiation “non-luminous heat”, back in the 1800s when such things were slowly being figured-out.
Sunshine is an example of radiation. Unlike conduction and convection, radiation has the distinction of being able to travel from its source straight through the vacuum of space. Thus, Solar radiation travels through that vacuum for some 150 million kilometres, to reach our planet at a near-constant rate. Some Solar radiation, especially short-wave UV light, is absorbed by our atmosphere. Some is reflected straight back to space by cloud-tops. The rest makes it all the way down to the ground, where it is reflected from lighter surfaces or absorbed by darker ones. That's why black tarmac road surfaces can heat up until they melt on a bright summer's day.

Figure 3: Heat haze above a warmed road-surface, Lincoln Way in San Francisco, California. May 2007. Image: Wikimedia Commons.
Energy balance
What has all of the above got to do with global warming? Well, through its radiation-flux, the Sun heats the atmosphere, the surfaces of land and oceans. The surfaces heated by solar radiation in turn emit infrared radiation, some of which can escape directly into space, but some of which is absorbed by the greenhouse gases in the atmosphere, mostly carbon dioxide, water vapour, and methane. Greenhouse gases not only slow down the loss of energy from the surface, but also re-radiate that energy, some of which is directed back down towards the surface, increasing the surface temperature and increasing how much energy is radiated from the surface. Overall, this process leads to a state where the surface is warmer than it would be in the absence of an atmosphere with greenhouse gases. On average, the amount of energy radiated back into space matches the amount of energy being received from the Sun, but there's a slight imbalance that we'll come to.
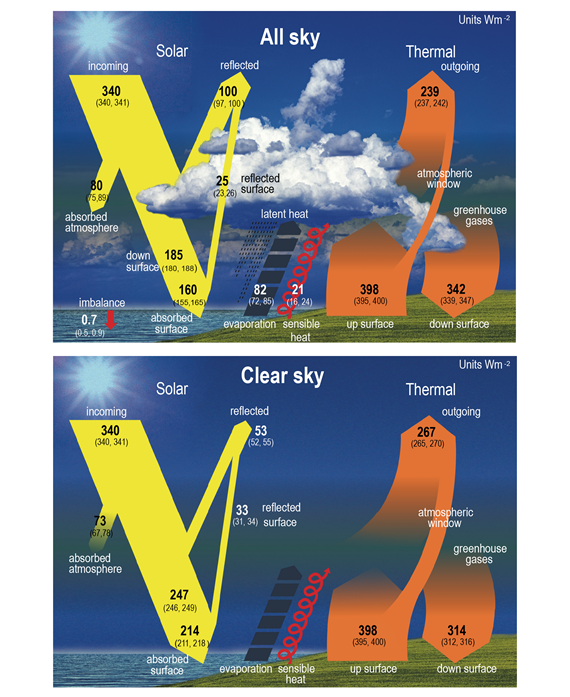
If this system was severely out of balance either way, the planet would have either frozen or overheated millions of years ago. Instead the planet's climate is (or at least was) stable, broadly speaking. Its temperatures generally stay within bounds that allow life to thrive. It's all about energy balance. Figure 4 shows the numbers.
Figure 4: Schematic representation of the global mean energy budget of the Earth (upper panel), and its equivalent without considerations of cloud effects (lower panel). Numbers indicate best estimates for the magnitudes of the globally averaged energy balance components in W m–2 together with their uncertainty ranges in parentheses (5–95% confidence range), representing climate conditions at the beginning of the 21st century. Figure adapted for IPCC AR6 WG1 Chapter 7, from Wild et al. (2015).
While the flow in and out of our atmosphere from or to space is essentially the same, the atmosphere is inhibiting the cooling of the Earth, storing that energy mostly near its surface. If it were simply a case of sunshine straight in, infra-red straight back out, which would occur if the atmosphere was transparent to infra-red (it isn't) – or indeed if there was no atmosphere, Earth would have a similar temperature-range to the essentially airless Moon. On the Lunar equator, daytime heating can raise the temperature to a searing 120OC, but unimpeded radiative cooling means that at night, it gets down to around -130OC. No atmosphere as such, no greenhouse effect.
Clearly, the concentrations of greenhouse gases determine their energy storage capacity and therefore the greenhouse effect's strength. This is particularly the case for those gases that are non-condensing at atmospheric temperatures. Of those non-condensing gases, carbon dioxide is the most important. Because it only exists as vapour, the main way it is removed is as a weak solution of carbonic acid in rainwater – indeed the old name for carbon dioxide was 'carbonic acid gas'. That means once it's up there, it has a long 'atmospheric residency', meaning it takes a long time to be removed.
Earth’s temperature can be stable over long periods of time, but to make that possible, incoming energy and outgoing energy have to be exactly the same, in a state of balance known as ‘radiative equilibrium’. That equilibrium can be disturbed by changing the forcing caused by any components of the system. Thus, for example, as the concentration of carbon dioxide has fluctuated over geological time, mostly on gradual time-scales but in some cases abruptly, so has the planet's energy storage capacity. Such fluctuations have in turn determined Earth's climate state, Hothouse or Icehouse – the latter defined as having Polar ice-caps present, of whatever size. Currently, Earth’s energy budget imbalance averages out at just under +1 watt per square metre - that’s global warming.
That's all in accordance with the laws of Thermodynamics. The First Law of Thermodynamics states that the total energy of an isolated system is constant - while energy can be transformed from one component to another it can be neither created nor destroyed. Self-evidently, the "isolated" part of the law must require that the sun and the cosmos be included. They are both components of the system: without the Sun as the prime energy generator, Earth would be frozen and lifeless; with the Sun but without Earth's emitted energy dispersing out into space, the planet would cook, Just thinking about Earth's surface and atmosphere in isolation is to ignore two of this system's most important components.
The Second Law of Thermodynamics does not state that the only flow of energy is from hot to cold - but instead that the net sum of the energy flows will be from hot to cold. To reiterate, the qualifier term, 'net', is the important one here. In the case of the Earth-Sun system, it is again necessary to consider all of the components and their interactions: the sunshine, the warmed surface giving off IR radiation into the cooler atmosphere, the greenhouse gases re-emitting that radiation in all directions and finally the radiation emitted from the top of our atmosphere, to disperse out into the cold depths of space. That energy is not destroyed – it just disperses in all directions into the cold vastness out there. Some of it even heads towards the Sun too - since infra-red radiation has no way of determining that it is heading towards a much hotter body than the Earth,
Earth’s energy budget makes sure that all portions of the system are accounted for and this is routinely done in climate models. No violations exist. Greenhouse gases return some of the energy back towards Earth's surface but the net flow is still out into space. John Tyndall, in a lecture to the Royal Institution in 1859, recognised this. He said:

As long as carbon emissions continue to rise, so will that planetary energy imbalance. Therefore, the only way to take the situation back towards stability is to reduce those emissions.
Update June 2023:
For additional links to relevant blog posts, please look at the "Further Reading" box, below.
Last updated on 29 June 2023 by John Mason. View Archives































 Arguments
Arguments



































MattJ @1451 & @1455.
@1451 you use words of mine @1436 to exemplify "many people here get on their high horses in defense of science — yet show they cannot even read well enough to do it."
@1455 you complain of "three simple questions" that have failed to be answered here.
It is worth considering the reasons for both these complaints as they have a common source. My input @1436 pointed to potential confusion created @1435 which is also where we find your "three simple questions."
Perhaps I should give three simple answers - (1) What is the "it" you are asking about? (2) Who do you think we are, Sky Dragon Slayers? (3) See (2).
And be mindful, MattJ, that @1435 there had already been a prior trail of alleged missed points and disputes over what you had actually written.
Seeking some semblence of sound thinking, we can look back to MattJ@1429 - "It does no good to quote the Second Law incorrectly, and then say, "it does not contravene the second law of thermodynamics" ... since you are still requiring radiating CO2 molecules in a -20C stratosphere to heat up an ocean layer that is on average above +20C ... it is still a violation of the "imaginary second law", but not of the law as Clausius really stated it." (Note my editing here makes things a whole lot clearer.)
Simply MattJ argues that the statement of the Second Law as presented in the SKS post is inadequate. He suggests using the WIkipedia version (from Clausius (1854) - an 1856 translation here) to overcome the inadequacies of the version used by the SKS post. Meeting a rebuff on this MattJ, you compound the confusion with comments that are pretty dire at describing your position and in detail packed with trollish statements.
Now, if your "it" in your first question @1435 encompassed the whole of that '@1429 statement' presented above, then there is sensible discussion to be had. For myself, I see a lot of scope for improving the post but I am not convinced that some pre-photon eighteenth century quote will expose the nonsensical cherry-picking of Sky Dragon Slayers and their ilk, however authoritative the quote. Do note in the quote from Wikipedia and its source, Clausius still talks throughout in terms of "the interchange of heat between two bodies of different temperatures" and, beyond the implications of the word 'interchange', never makes clear that he talks of 'net heat flow.'
However to argue the "it" actually is the whole statement @1429 would be rather difficult as it requires some strange interpretation of the words used.
So my response to MattJ @1451 is this - It is not my reading of the words that is at fault but instead the fault lies with the writing of the words I am being expected to read.
MA Roger, while the translation doesn't explicitly use the phrase "net flow of heat", I think it is implicitly clear in the translation of the first edition of Clausius' textbook in the footnote mentioned in my post at 1146:
The only way for this to be satisfied (in the absence of "some other change") is for the net flow of heat to be from the warmer to the cooler body.
Dikran Marsupial @1477.
The passage you found in the Tyndall translation (actually @1446 although the link does the job) certainly makes the two-way flow explicit. It is perhaps still a bit obscure being within a footnote but I think if you also point out that the statements of the law nowhere say they aren't talking about net heat flow, then the footnote would be difficult to refute.
Also I wonder if describing the changing size of the vibrations of atoms in a solid that result in transmited heat by conduction may also be useful to show up how brainless the 'no heat shall pass from cold to hot' interpretation really is. Just as the nutters are arguing that by magic photons don't get fired at warmer objects, they also have to be arguing that vibrations only excite adjacent atoms in the direction of net heat flow. How can a vibration only act in one single direction? A mind-boggling requirement.
First of all, many thanks for all your hard work! I occassionally get involved in the debate on AGW theory and your site is a great resource for sorting out the arguments and debunking the junk science streaming out of the "denialist" camp - they are like an army of Duracell bunnies and any discussion tends to quickly turn into a crap-storm of half-truths, contradictions and downright lies. Without sites such as skepticalscience it would be impossible to keep up!
Now most of the arguments they tend to throw up are just regurgitations of old misunderstandings, willful or otherwise, and as such I'm usually able to answer back pretty quickly, but recently something's come up which has me scratching my head. I'm referring to the Donhoe et al. paper from the 10th of November this year; "Shortwave and longwave radiative contributions to global warming under increasing CO2". If I understand it correctly, Donohoe & co show that long-wave "trapping" by CO2 is not the main driver behind global warming, and that short-wave absorbtion by water vapour (and reduced albedo) is the real cause of long term warming. So far so in accordance with observation: the earth is getting warmer and it's caused by an increase in its ability to retain heat from the sun. Climate change 101. But what I find extremely confusing is the claim that warming from CO2 is only a temporary effect; their models show that in as little time as a decade the warming effect of CO2 is balanced out by an increase in long-wave radiation back to space, "cancelling out" the warming effect. I have to admit this is complete news to me; I was always under the impression that the CO2 content (and other greenhouse gases) of the atmosphere of any planet was directly linked to that planet's temperature, and that all other things being equal, the planet with the higher greenhouse gas concentrations would be the warmer one (e.g. Venus). Instead I see claims like this:
"When CO2 is first added, it does act as a blanket, trapping long-wave infrared energy coming off the Earth. The atmosphere then emits less of this long-wave radiation to space because the upper atmosphere is cooler than the Earth's surface, just as the top of your blanket is cooler than your body. But the Earth gradually heats up under this blanket, and hotter objects emit more long-wave radiation, so within about a decade the effect of adding the thicker blanket has been canceled by the warmer body emitting more energy." (source)
My admittedly limited understanding of thermal equilibrium tells me that yes, add quantity X of CO2 and you will get an increase in the ability to retain heat; the planet will warm up to temperature Y, at which point long-wave radiation back to space will balance out the heat-trapping ability, and temperature will stop rising. Add more CO2 (or other greenhouse gases) and the temperature will notch up further before again reaching equilibrium. Two objects at the same distance from a radiation source, one black, one white, the black object will be warmer as it absorbs more of the incoming radiation - and as a consequence it will also emit more long-wave radiation. Both objects are at thermal equilibrium, but one is warmer than the other due to it's ability to capture more of energy. But then I see this:
"Most of the study's simulations involved a one-time addition of carbon dioxide into the atmosphere. One scenario simulated continuously increasing CO2, as is happening now - in that case, the long-wave radiation effect lasted about 20 years before the shortwave effect took over."
What!? Unless my reading comprehension has suffered a sudden dramatical reduction (a distinct possibility!) it seems they are saying that even if you keep increasing the CO2 content, the effect will eventually (20 years?) be cancelled out and (apart from the warming from short-wave radiation) the planet in question would return to its earlier temperature. This just doesn't make any sense to me; if CO2 is able to "trap" long-wave radiation, surely if you keep increasing the CO2 level, the "trapping" ability will also continue to increase? I would greatly appreciate if someone could shed some light on this.
Lomax, when the first quotation says, "...within about a decade the effect of adding the thicker blanket has been cancelled by the warmer body emitting more energy", the 'effect' in question is the amount of outgoing long wave radiation. The planet (i.e. "the warmer body") is still hotter... it is just that the increased heat eventually leads to the radiation flows in and out equalizing. The planet isn't going to "return to its earlier temperature" as you say... just to it's earlier OLR rate, which is happening precisely because the temperature has increased.
"This just doesn't make any sense to me; if CO2 is able to "trap" long-wave radiation, surely if you keep increasing the CO2 level, the "trapping" ability will also continue to increase?"
Yes, but as you 'trap' more long wave radiation within the climate system that causes/is heat buildup... which causes/is even more long wave radiation emission, until eventually the extra amount being emitted equals the extra amount being trapped and the total output returns to its original level.
Think of it as a lake with one stream flowing in and three streams flowing out. If you block off one of the outflow streams (i.e. add CO2 to reduce OLR) then the amount coming in from the inflow stream (i.e. radiation from the Sun) now exceeds the amount going out and the water level rises (i.e. the Earth gets hotter). However, as the water level rises that causes it to flow out the two remaining outflow streams faster (i.e. the amount of longwave radiation increases) and eventually the lake water level stops rising when the inflow and outflow rates are again in balance (i.e. the temperature stops increasing when incoming and outgoing radiation again match).
For the record, none of that is actually the 'result' found by this study... that's all basic global warming. What this study is arguing that is 'new' is that the warming effect of increasing CO2 will very quickly be overwhelmed by the feedback warming effect of a darkening planetary surface (e.g. from melting snow and ice). I'm not sure I buy that, for one thing it's a model result which doesn't seem to jibe with paleoclimate studies, but if anything it actually suggests that warming will be much greater than expected.
@CBDunkerson: Great, many thanks for clarifying; I guess that summary was just poorly written - as was my post, on second read-through :) I'm relieved to hear that my understanding of thermal equilibrium was basically correct and that the greenhouse effect works the way I thought (though I'm not sure relieved is the correct word here). Indeed it does sound worrying that the feedback from reduced albedo and increased water vapour levels might be greater than previously thought - let's hope they are proven wrong!
I've italicised your words. My rebuttal follows. Please explain.
They radiate most of the heat that is received from the sun, so the average temperature of the Earth stays more or less constant.
All energy the Earth receives from the sun is returned to space. That’s what the Law of conservation of Energy and the Climate Energy Balance states. The atmosphere cools the Earth. It does not add heat. The surface of the moon is +200F. Why, because there's not convection to draw the heat away.
Greenhouse gases trap some of the escaping heat
C02 does NOT “Trap” heat. Kirchhoff's law of thermal radiation clearly states that a body’s ability to absorb heat energy is equal to its ability to emit that heat energy.
So the greenhouse gases make the Earth warmer - like a blanket conserving body heat - and voila, you have global warming. When you wrap yourself in a blanket, the loss of heat is reduced, some is retained at the surface of your body, and you warm up. You get warmer because the heat that your body is generating cannot escape as fast as before.
Heat moves via convection, conduction or radiation. Gas-convection, Solid-conduction, Vacuum-radiation.
A blanket is NOT the same as a trace gas like C02. One is a solid and one is a gas. A solid object like a blanket will impose on the rate of heat loss through the loss of convection. The blanket is NOT adding heat to one’s body. If, instead of a blanket, we set the environment to a saturated C02 level of 100%, the heat loss would be the same as if it was at zero. Your explanation using a blanket is ridiculous.
"Heat generally cannot flow spontaneously from a material at lower temperature to a material at higher temperature."
It should read…. "Heat CANNOT flow spontaneously from a material at lower temperature to a material at higher temperature." The Laws of Thermodynamics are LAWS not theory.
[JH] Please keep it civil.
[PS] You cannot refute an argument when you dont understand how it works. Short of opening a textbook on radiative physics, I would strongly suggest that you look over Science of Doom here and here which takes in stages and spends whole articles on your objections. Hopefully you will admit that if an experiment produces a different result from your perception of a theory, then your perception is wrong.
the master... "The surface of the moon is +200F."
Sorry, but the average temperature of the moon isn't 200°F. That's rather an absurd cherry pick.
the master... "C02 (sic) does NOT 'Trap' heat." [Mods should probably warn about all caps.]
CO2 absorbs and reradiates IR, thus causing more heat to remain in the climate system. That's what people mean by this phrase.
the master... "A blanket is NOT the same as a trace gas like C02 (sic)."
"A blanket" is a metaphor. No metaphor is intended to be exactly like anything, otherwise it wouldn't be a metaphor. As a metaphor, a blanket is a good way to explain how greenhouse gases work.
[Again with the all caps "not."]
The Master:
Interesting choice of names.
Answering your points in order, your words in italics:
"All energy the Earth receives from the sun is returned to space." Some energy is absorbed by the surface and not returned to space for a period of time. That can range from a day or two up to centuries. A great deal of ice has melted from Greenland and the Antarctic the past few years. The heat to melt that ice came from the sun. It will not be returned for centuries (or more likely thousands of years) when the ice freezes again. On average over a very loong period of time all the energy is re-emitted but for shorter times (like the life of a human) some is absorbed and raises the temperature of the Earth. At other times more energy is emitted than absorbed as the Earth goes into a glacial period.
The surface of the moon is .+200C during the day but it is -200C at night The average temperature is not 200F as you claim. The temperature of the moon is as predicted by scientists. The colder average temperature of the Moon shows that the Earth is heated by greenhouse gases. This has been known by scientists since 1850.
" Kirchhoff's law of thermal radiation clearly states that a body’s ability to absorb heat energy is equal to its ability to emit that heat ." At 5 kilometers above the surface the temperature is about 30C less than at the surface. If the surface emits IR radiation proportional to its temperature some of that energy will be absorbed by CO2 at 5 km. Since the CO2 is colder than the surface it cannot radiate the energy outward as fast as it absorbs it, even though it follows Kirchhoff's law. You need to pay more attention to the temperature of the emitting and absorbing surfaces. Mistakes like this make it appear that you have not thought through the physics enough yet.
"If, instead of a blanket, we set the environment to a saturated C02 level of 100%, the heat loss would be the same as if it was at zero." Many videos on You Tube show that bottles filled with CO2 heat up faster than bottles filled with air. The heat loss is lower when the atmosphere is CO2.
You have me with an example of heat flowing from a cold area to a warmer area, but that statement is not used in the argument so it is moot.
In general it is easier to discuss your misunderstandings of the scientific arguments if you limit your arguments to one problem at a time. Once that issue is resolved we can move on to the next misunderstanding. When there is a long list like yours the replies become difficult to read.
tm @ 1482:
200 Fahrenheit is approximately 366.5 Kelvin. To put your claim into context, the most thorough scientific examination of the surface temperature of the Moon says:
In other words, your estimate of the Moon's temperature over estimates the mean equatorial temperature by 155 K (279oF). It overestimates the mean lunar surface temperature by much more.
You will also note that the lunar mean equatorial temperature is about 72 K less than the Earth's Global Mean Surface Temperature. As the equator of the Moon has the warmest mean temperature of any latitude, it follows that the Global Mean Surface Temperature of the Moon is much more than 72 K less than the Earth's. That is despite the fact that the Moon's albedo of 0.136 is less than half of that of the Earth's. It follows, even by your reasoning, that the Earth's atmosphere heats it relative to what it would be as an airless body.
As it happens, the major part of that warming is due to the thermal inertia of the atmosphere, and the oceans (in particular), along with the poleward heat transport by both, results in much more equal temperatures. However, the Earth's Global Mean Surface Temperature is about 33 K warmer than would be possible with its albedo, even if its surface were a uniform temperature. That means the combined effect of the atmosphere (radiative and convective energy transfer) warms the Earth's surface. As it happens, it has been shown that the radiative effect warms it, while convection cools the surface relative to what it would be with the radiative effect (ie, the greenhouse effect) alone.
Further to tm @ 1482
The term "heat" is used ambiguously. Some people use it to mean the thermal energy. Used with that meaning, it is unambiguously the case that thermal energy (in the form of IR radiation) can flow from a colder body to a warmer body, although more must flow in the reverse direction.
The other (possibly more scientifically accurate) meaning is "net thermal energy flow", under which meaning it is unambigously the case that "net thermal energy flow" must be from the warmer body to the colder body (something that can be deduced from the preceding paragraph).
Needless to say, the sentence you find offensive uses the first, and more common in popular usage, meaning.
Under either definition, the 2nd Law of thermodynamics is no bar to the Greenhouse Effect which predicts that net energy flow will be from the Sun to the surface, and then surface outwards. This can be clearly seen in the standard model used to teach the mathematics of the Greenhouse effect:
You will notice that the downward IR radiation at each level is less than the upward IR radiation from the level below, so that the net energy flow is upward. You will also notice that, as the model shows an equilibrium condition, the net IR energy flow between each level is an upward flow equal to the downwar flow from solar radiation. However, to maintain this condition (which conforms with all energy conservation laws), the ground level upward flux must be (in this example), four times the incoming flux from the solar radiation, implying a very strong greenhouse warming. It is only able to do this in compliance with the laws of thermodynamics because of the downward IR flux from the layer above.
Note: this is just a toy model used to illustrate imortant concepts, and introduce a very basic level of the maths involved. It is in no way meant to represent a real situation. In real situations, atmospheres do not conveniently divide themselves into layers like that, and convection is a crucial element. However, in real life the Earth's surface is warmer than can be accounted for by solar radiation and the the Earth's albedo alone, and the net energy flow upwards from all sources matches the downward energy flow from the incoming solar radiation at all levels (although not all levels are shown below):
That is, there is a greenhouse effect, but it is in complete confirmation with the laws of thermodynamics (which is no surprise given that it was predicted by some of the key figures in determining those laws.)
Rob Honeycutt @1485,
You say the descrption "blanket" is but a metaphor. I would disagree. The convection within the atmosphere is very very slow. How else could it be? Convection contributes directly only 4% of the surface cooling (as conveniently illustrated @1488 above). And that is because the circulation of the atmosphere is very slow. Outside cyclones it takes something like two weeks for a parcel of air to move from surface to tropopause and back. The atmosphere is not static but neither is a greenhouse hermetically sealed. And blankets are far from air-tight. Just like with the planet surface and the GH effect, a blanket's main mode of operation is not preventing cooling convection but in trapping radiant heat loss. So I would suggest that both 'greenhouse' and 'blanket' are analogies and not metaphors.
MA Rodger @1489, the energy balance diagram only shows energy movement between realms - ie, from the surface to the atmosphere, or from the atmosphere to the surface. It does not show energy transfer within the atmosphere itself. For that reason, the figure is not a good guide for estimated what Global Mean Surface Temperature (GMST) would be like in the absence of convection.
A better guide is Fig 4 from Manabe and Strickler (1964):
As you can see, from their model, an absence of latent heat transfer (ie, dry-adiabatic lapse rate) would lift GMST by about 10oC, while the complete absence of convection would lift it by about 45oC relative to current conditions. As the greenhouse effect on Earth raises GMST by over 33oC, the presence of convection cools the Earth by over 50% of the temperature increase that would occur from a greenhouse effect without convection.
Eliminating latent heat transfer within the atmosphere by the condensation of water would eliminate just under 25% of the greenhouse effect coupled with convection. That is an esoteric figure, however, given that 75% of the greenhouse effect is from water vapour and clouds. Consequently the combined effect (greenhouse and lapse rate) of water vapour in the atmosphere is to warm the Earth; although at a lower GMST it might be to cool it given the reduced greenhouse effect but near constant cloud albedo effect.
MA Rodgers... I suppose that would be a function of how you referred to it. You can make it a simile, metaphor or an analogy. I think TM was attempting to dismiss it on a more literal basis and that, I believe, was deliberately missing the point.
Rob Honeycutt @1491,
I agree that when explaining the GH effect, at a minimum there is a willful prejudice being employed when an immutable counter-argument wielded against both "blanket" and "greenhouse" as an analogy (or metaphor or whatever) is of the form as presented @1482 (ie - "One is a solid and one is a gas.").
But my thought is to break that immutable nonsense rather than live with it. Thus my position trying to revitalise both the "blanket" and the "greenhouse" as strong analogies for the GH effect.
Thus I would argue as follows.
Note the follow-on comment @1482 "A solid object like a blanket will impose on the rate of heat loss through the loss of convection." A blanket does not trap air anything like as well as the lower atmosphere. The plume of hot gas passing through a blanket is travelling at quite a rate (as this test shows - note 400ft/min =2m/s) And greenhouses are not hermetically sealed but are actually far more leaky that the lower atmosphere, even commercial ones. The lower atmosphere retains parcels of air for a week or more. The same cannot be said for either any blanket or any greenhouse.
And this slow action of the atmosphere circulation is probably best explained by the planet Earth being so big and tha atmosphere so shallow. In the tropics a latitudinal band measuring say 1,000 km is heated and so it is wanting to rise up and displace the cooler air above it. This creates a Walker Cell which will flow away from the tropics at the top of the atmosphere where it cools by radaition into space and so cooled drops back down to the surface outside the tropics. The problem for the "big convetion" argument is that the longitudinal winds at the top of the atmosphere (and the balancing ones in the lower atmosphere) are much less than 10m/s. This speed of wind is the result of the upward convetion flow from the tropics but the <10m/s represents a massive acceleration from the vertical convetion speeds - because comparatively the tropics are very broad but the atmosphere is rather shallow. If the vertical motion feeding this <10m/s is 1,000km wide, the upward speed has to be <0.02m/s which means the air in any "big convetion" cooling circulation will take >600,000 seconds or seven days to reach the top of the atmosphere. Radiation, on the other hand, is flying about at the speed of light. So it's not much of a contest, is it? Thus the values of the various fluxes in the Earth Energy Balance diagram as shown @1488 show very small quantities of convection in operation.
Now this last bit referring to the diagram is a bit of a throw-away remark. The diagram in its original form did describe the sensible heat flux as "thermals" (eg in Trenberth et al 2009 figure 1) which is wrong. But I'm not sure it is entirely wrong.
Tom Curtis @1490,
The Manabe & Strickler model presents interesting findings but I'm not sure that I would accept them without a fight. Likewise, the figure of 5% convection contribution deriving from the figure presented @1488 is also not well established. (The 5% is more the value for the conduction of sensible heat from surface to atmosphere and certainly is not a good value to rely on.)
While the Manabe & Strickler non-convection calculation of atmospheric temperature profile is a useful calculation, I do not see it as the final word. Firstly, it is being compared with two other profiles which pre-suppose the lapse rate. Thus the model Manabe & Strickler were developing is really just testing their model above the troposphere. So is the non-convection version a fair one? It assumes only a single value for the H2O profile which may not be (probably isn't) a good choice for a global average. Indeed, what model should be set up to calculate a non-convective atmospheric GH effect? That is the start point. I think you would have to freeze representative GHG levels (& presumably cloud) over the whole globe to calculate it. I would suggest that the result would show that it is the holes in the H2O blanket that are responsible for a very large portion of the energy balance. This is why the Wanabe & Strickler graph without such a hole shows the large increase in temperature for non-convection at low altitudes where in their modelled H2O is high.
And a quick back-of-fag-packet argument. If we look at average surface temperatures, if half the cooling were convective, would we see a significant temperature differential between equator & 30 degrees? If we look at TOA outward IR (eg Trenberth & Stepaniak (2004) fig 3a&b) would there be a sign of a cooler upper atmosphere above the outer limit of the Hadley cells? Of course, what we see is more LR at the outer limits and less over the tropics which suggests it is the GH effect which continues to dominate the outward energy budget through the thickness of the troposphere with the dry atmosphere sinking in the gentle Hadey cell flow allowing big energy fluxes to flow out from the planet, averaging perhaps roughly 270W/sq m. Over the equator the H2O GH effect keeps that energy flux down to say 225W/sq m. Of course, all this very much first-cut response to the Manabe & Stickler model.
This does not seem a good explanation for how greenhouse theory is not at odds with the second law of thermodynamics. A body will lose heat at a rate relative to the surrounding temperature. If it is much colder and your body loses heat quickly you feel cold. The blanket does slow the transfer of heat from your warm body to the cold atmosphere, making you feel warmer, or more correctly less cold. You do not actually get hotter just colder slower, whereas greenhouse theory States the earth gets hotter, not colder slower.
[PS] This is the longest thread at Sks and it seems to be because people have simultaneously have a very poor grasp of thermodynamics and a great reluctance to improve it. Science of Doom have an excellent series on the textbook basics as well as good article on Second Law. But please be clear that if you are going to insist on a description of thermodynamics that is not in accordance with experimental results, then this is not site for you.
I suspect the informal article with the jacket analogy, the intermediate article and the video all help resolve any confusion for most people. Actually seeing pyrgeometers measuring downward infra-red radiation adds to the knowledge of how we know. Intuition can be more important to non-experts than logic. On which subject, the intermediate page is very good, but my intuition tells me that a big atmospheric window from 10 to 13 µm should allow more than 40 Wm¯² of surface radiation (about 10% of it) to directly escape to space. What's the best way of doing that calculation?
Maybe rather than starting with the overall behaviour, it is good to show that each component in the system is behaving according to physical laws. Would presenting the simple equations for conduction and for radiation help, as it would clarify that heat transfer is proportional to temperature difference in the case of conduction, but not in the case of radiation? Some people seem to disbelieve Stefan-Boltzmann, asserting that an object somehow knows it should cease to radiate towards a warmer object.
Anyway, I wanted to mention to more 'grey literature' online resources helping to clarify the confusion, rather than textbooks. Science of Doom provides basics (mentioned in response to 1494) for those willing to go through them, but goes beyond them: here's a 2017 challenge to anyone arguing against the greenhouse effect on thermodynamic grounds: explain your own view numerically.
More simple, intuitive understanding of the principles is provided by Eli Rabbett's Green Plate Effect. In particular, here's a video by izen.
[PS] Thank you. Those are all good resources.
I'm fairly new to this site and just catching up on a lot of articles and comment streams. Is this myth still used? The way I explain it to laymen is...
The CO2 greenhouse effect was discovered in the 1890's. Much of its fundamental underpinnings were discovered and confirmed with Cold War era military research. So I find it exceptionally hard to believe that this theory was studied for ~90 years before the existence of the IPCC and nobody noticed that it violated basic laws of physics.
AFT, anyone who thinks seriously about it undertands that thermodynamics are not violated. This argument is just part of larger campaign undertaken by some actors because they know where the morally defensible position is and that people will in their majority adopt the morally right position if there is no doubt about it.
The depth of the denial is compounded by numerous factors. Some scientists, like G&T, are unscrupulous enough to write such nonsense. The general population is science illiterate and innumerate enough to buy into it. The ambient attitude that anyone is free to have whatever opinion they choose is stretched to the point that it implies said opinion has validity. The overall anti elite and anti intellectual sentiment has been cultivated by crooks purely for the fostering of their financial interest.
There is little to gain by arguing with those who go for the 2nd law argument; they are ready to cling to any straw, no matter how feeble and likely won't be convinced by any level of reasoning or evidence. Look how long this thread is. Waddle in it if you want, it's saddening. Almost 1500 post devoted to the least valid "skeptic" argument of all. It says something.
So I just encountered this one, which appears to be a variant of "AGW violates basic physics"...
"It ends up being trivially easy to understand that gravity, not spectrum , is why and by how much bottoms of atmospheres are hotter than their tops. See my website which includes links to my Heartland Inst talk showing the impossibility of explaining Venus's surface temperature , 400c hotter than what it absorbs from the Sun, as a spectral effect."
[PS] Doesnt like a 2nd law argument. More like the Postma nonsense. Surface temperature is end result of all relevant physics including GHE. Actually I think the author is Bob Armstrong who has some times demonstrated his grasp of physics here. Try https://www.google.com/search?q=bob+armstrong+site%3Askepticalscience.com
Greenhouse Gas Theory does not violate the Second Law of Thermodynamics
Attempts have been made to discredit the GHG theory by claiming that it violates the Second Law of Thermodynamics and so the theory cannot be valid. I have seen some rebuttals on this site, but I was not convinced; so I now offer my own approach which I hope will be helpful.
The claims of violation are based on the idea that heat cannot flow from a cold object to a hotter one. In general, with heat transfer by conduction and convection, this idea is very true. But the Second Law does not actually state that idea. In fact, it deals with another property of the system, known as entropy, which is the degree of disorder of the system. If the system is very “tidy”, the disorder is small, and so is the entropy. Great “untidiness” means high entropy.
The Second Law of Thermodynamics states that the entropy of a system will increase.
It does not mention Heat.
In the case of heat transfer, it is readily seen that the Law is obeyed by conduction and convection between objects in contact. Higher temperatures mean that the atoms and molecules are moving/vibrating more rapidly and to a greater extent than at colder temperatures, and so have high entropy. Some of the greater movements in the hot object can be passed into the cold object, so increasing the overall degree of disorder, or entropy, of the system. But, the reverse cannot happen, according to the Law, because the colder object has smaller entropy.
Therefore, heat can flow from hot to cold, but not from cold to hot. And it is only for conduction and convection transfer.
This leaves us to consider heat transfer by radiation.
This is the method by which heat is claimed to flow, according to the GHG theory, from the carbon dioxide in the atmosphere to the Earth’s surface. But the atmosphere is cooler than the Earth’s surface, so how can this happen? Does it not violate the Second Law? And this is the big problem the GHG theory has to overcome.
Not a problem. Consider photons of infrared energy emitted downwards from carbon dioxide molecules in the atmosphere. These are neat little wave-packets of electromagnetic energy, and have no charge. Very neat and tidy. The entropy involved is small because they are not continually in vibrational contact with each other, as are the atoms in a solid, or the molecules in a gas. Once emitted, they will continue in their motion until they are absorbed by the Earth’s surface, (although some may collide with molecules in the air). Upon absorption, the energy given to the surface atoms and molecules causes them to increase vibration and movement, and so the entropy, the degree of disorder, increases. OK, and the surface gains energy and so the temperature rises.
The entropy increases. This is in agreement with the Second Law. No violation, but the temperature of the surface of the Earth also increases. This is what the GHG theory says.
AEBanner @1499 ,
Well said. Including your description of entropy as "untidiness".
This particular thermodynamic "Law" is a source of endless trouble to some people, because (as you have said) they do not look at the basic physical entities involved. Instead, they stand back and try to view the universe as ruled and directed by "Laws" . . . laws which are actually simply abstract conceptions in the human mind. It's all a very Nineteenth Century religion-like viewpoint. Very pre-Einstein, pre-quantum-mechanics way of thinking. Mistaking the concept for the reality. ( Can we blame Plato for this? )