Is the CO2 effect saturated?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
The notion that the CO2 effect is 'saturated' is based on a misunderstanding of how the greenhouse effect works. |
|||||||
Climate Myth...
CO2 effect is saturated
"Each unit of CO2 you put into the atmosphere has less and less of a warming impact. Once the atmosphere reaches a saturation point, additional input of CO2 will not really have any major impact. It's like putting insulation in your attic. They give a recommended amount and after that you can stack the insulation up to the roof and it's going to have no impact." (Marc Morano, as quoted by Steve Eliot)
At-a-Glance
This myth relies on the use (or in fact misuse) of a particular word – 'saturated'. When someone comes in from a prolonged downpour, they may well exclaim that they are saturated. They cannot imagine being any wetter. That's casual usage, though.
In science, 'saturated' is a strictly-defined term. For example, in a saturated salt solution, no more salt will dissolve, period. But what's that got to do with heat transfer in Earth's atmosphere? Let's take a look.
Heat-trapping by CO2 in the atmosphere happens because it has the ability to absorb and pass on infra-red radiation – it is a 'greenhouse gas'. Infra-red is just one part of the electromagnetic spectrum, divided by physicists into a series of bands. From the low-frequency end of the spectrum upwards, the bands are as follows: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Gamma rays thus have a very high-frequency. They are the highest-energy form of radiation.
As our understanding of the electromagnetic spectrum developed, it was realised that the radiation consists of particles called 'photons', travelling in waves. The term was coined in 1926 by the celebrated physicist Gilbert Lewis (1875-1946). A photon's energy is related to its wavelength. The shorter the wavelength, the higher the energy, so that the very high-energy gamma-rays have the shortest wavelength of the lot.
Sunshine consists mostly of ultraviolet, visible light and infra-red photons. Objects warmed by the sun then re-emit energy photons at infra-red wavelengths. Like other greenhouse gases, CO2 has the ability to absorb infra-red photons. But CO2 is unlike a mop, which has to be wrung out regularly in order for it to continue working. CO2 molecules do not get filled up with infra-red photons. Not only do they emit their own infra-red photons, but also they are constantly colliding with neighbouring molecules in the air. The constant collisions are important. Every time they happen, energy is shared out between the colliding molecules.
Through those emissions and collisions, CO2 molecules constantly warm their surroundings. This goes on all the time and at all levels in the atmosphere. You cannot say, “CO2 is saturated because the surface-emitted IR is rapidly absorbed”, because you need to take into account the whole atmosphere and its constant, ongoing energy-exchange processes. That means taking into account all absorption, all re-emission, all collisions, all heating and cooling and all eventual loss to space, at all levels.
If the amount of radiation lost to space is equal to the amount coming in from the Sun, Earth is said to be in energy balance. But if the strength of the greenhouse effect is increased, the amount of energy escaping falls behind the amount that is incoming. Earth is then said to be in an energy imbalance and the climate heats up. Double the CO2 concentration and you get a few degrees of warming: double it again and you get a few more and on and on it goes. There is no room for complacency here. By the time just one doubling has occurred, the planet would already be unrecognisable. The insulation analogy in the myth is misleading because it over-simplifies what happens in the atmosphere.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
This myth relies on the use of a word – saturated. When we think of saturated in everyday use, the term 'soggy' comes to mind. This is a good example of a word that has one meaning in common parlance but another very specific one when thinking about atmospheric physics. Other such words come to mind too. Absorb and emit are two good examples relevant to this topic and we’ll discuss how they relate to atmospheric processes below.
First things first. The effect of CO2 in the atmosphere is due to its influence on the transport of 'electromagnetic radiation' (EMR). EMR is energy that is moving as x-rays, ultraviolet (UV) light, visible light, infrared (IR) radiation and so on (fig. 1). Radiation is unusual in the sense that it contains energy but it is also always moving, at the speed of light, so it is also a form of transport. Radiation is also unusual in that it has properties of particles but also travels with the properties of waves, so we talk about its wavelength.
The particles making up radiation are known as photons. Each photon contains a specific amount of energy, and that is related to its wavelength. High energy photons have short wavelengths, and low energy photons have longer wavelengths. In climate, we are interested in two main radiation categories - firstly the visible light plus UV and minor IR that together make up sunshine, and secondly the IR from the earth-atmosphere system.
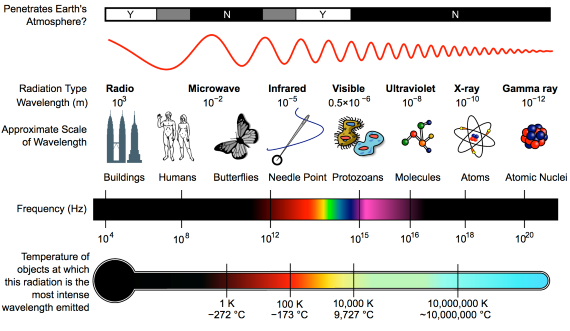
Fig. 1: diagram showing the full electromagnetic spectrum and its properties of the different bands. Image: CC BY-SA 3.0 from Wikimedia.
CO2 has the ability to absorb IR photons – it is a 'greenhouse gas'.So what does “absorb” mean, when talking about radiation? We are all familiar with using a sponge to mop up a water spill. The sponge will only absorb so much and will not absorb any more unless it's wrung out. In everyday language it may be described, without measurements, as 'saturated'. In this household example, 'absorb' basically means 'soak up' and 'saturated' simply means 'full to capacity'. Scientific terms are, in contrast, strictly defined.
Now let's look at the atmosphere. The greenhouse effect works like this: energy arrives from the sun in the form of visible light and ultraviolet radiation. A proportion reaches and warms Earth's surface. Earth then emits the energy in the form of photons of IR radiation.
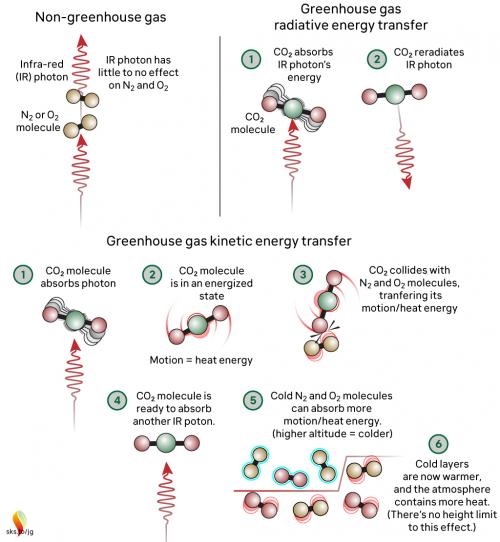
Greenhouse gases in the atmosphere, such as CO2 molecules, absorb some of this IR radiation, then re-emit it in all directions - including back to Earth's surface. The CO2 molecule does not fill up with IR photons, running out of space for any more. Instead, the CO2 molecule absorbs the energy from the IR photon and the photon ceases to be. The CO2 molecule now contains more energy, but that is transient since the molecule emits its own IR photons. Not only that: it's constantly colliding with other molecules such as N2 and O2 in the surrounding air. In those collisions, that excess energy is shared with them. This energy-sharing causes the nearby air to heat up (fig. 2).
Fig. 2: The greenhouse effect in action, showing the interactions between molecules. The interactions happen at all levels of the atmosphere and are constantly ongoing. Graphic: jg.
The capacity for CO2 to absorb photons is almost limitless. The CO2 molecule can also receive energy from collisions with other molecules, and it can lose energy by emitting IR radiation. When a photon is emitted, we’re not bringing a photon out of storage - we are bringing energy out of storage and turning it into a photon, travelling away at the speed of light. So CO2 is constantly absorbing IR radiation, constantly emitting IR radiation and constantly sharing energy with the surrounding air molecules. To understand the role of CO2, we need to consider all these forms of energy storage and transport.
So, where does 'saturation' get used in climate change contrarianism? The most common way they try to frame things is to claim that IR emitted from the surface, in the wavelengths where CO2 absorbs, is all absorbed fairly close to the surface. Therefore, the story continues, adding more CO2 can’t make any more difference. This is inaccurate through omission, because either innocently or deliberately, it ignores the rest of the picture, where energy is constantly being exchanged with other molecules by collisions and CO2 is constantly emitting IR radiation. This means that there is always IR radiation being emitted upwards by CO2 at all levels in the atmosphere. It might not have originated from the surface, but IR radiation is still present in the wavelengths that CO2 absorbs and emits. When emitted in the upper atmosphere, it can and will be lost to space.
When you include all the energy transfers related to the CO2 absorption of IR radiation – the transfer to other molecules, the emission, and both the upward and downward energy fluxes at all altitudes - then we find that adding CO2 to our current atmosphere acts to inhibit the transfer of radiative energy throughout that atmosphere and, ultimately, into space. This will lead to additional warming until the amount of energy being lost to space matches what is being received. This is precisely what is happening.
The myth reproduced at the top – incorrectly stating an analogy with roof insulation in that each unit has less of an effect - is misleading. Doubling CO2 from 280 ppm to 560 ppm will cause a few degrees of warming. Doubling again (560 to 1130 ppm) will cause a similar amount of additional warming, and so on. Many doublings later there may be a point where adding more CO2 has little effect, but recent work has cast serious doubt on that (He et al. 2023). But we are a long, long way from reaching that point and in any case we do not want to go anywhere near it! One doubling will be serious enough.
Finally, directly observing the specific, global radiative forcing caused by well-mixed greenhouse gases has - to date - proven elusive. This is because of irregular, uncalibrated or limited areal measurements. But very recently, results have been published regarding the deep reinterrogation of years of data (2003-2021) from the Atmospheric Infrared Sounder (AIRS) instrument on NASA's Aqua Satellite (Raghuraman et al. 2023). The work may well have finally cracked the long-standing issue of how to make finely detailed, consistent wavelength-specific measurements of outgoing long-wave radiation from Earth into space. As such, it has opened the way to direct monitoring of the radiative impact (i.e. forcing + feedback) of greenhouse gas concentration changes, thereby complimenting the Keeling Curve - the longstanding dataset of measured CO2 concentrations, down at the planet's surface.
Note: Several people in addition to John Mason were involved with updating this basic level rebuttal, namely Bob Loblaw, Ken Rice and John Garrett (jg).
Last updated on 31 December 2023 by John Mason. View Archives































 Arguments
Arguments





































Well if you want to push the sleeping bag analogy, increasing the insulation strength will always increase the temperature (Fourier Law). However, you cannot push the analogy too far. The key to understanding the saturation argument is undertstanding the importance of the temperature profile to absorption. The Modtran outputs you posted looked fine so I only assume you are not understanding how they work. Try this discussion.
Ultimately though, it is great to try and understand the impact of the equations, but it is unreasonable to deny the lab and field tested solutions. When you can directly measure the increase in radiation from added CO2, then obviously the effect is not saturated.
Pringlesx:
You raise an interesting question. Fortunately, the answer can be easily calculated.
According to this web site, insulation r values add. I am a cheap SOB so I buy cheap R=1 m2 T/W sleeping bags (metric units). Since I have 99 cheap sleeping bags the total R value is 99 m2 T/W.
To calculate the internal temperature we use the equation:
Watts = m2 x deltaT devided by R
My sleeping bags are 2 meters long and 2 meters around so they have a surface area of 4 meters square. A human body generates about 100 watts of energy while sleeping.
Plugging the data into the equation I find the temperature difference after you stay in the bags to equilibrium is 100W x 99R devided by 4 = about 2475 degrees C. If it is -50C outside than the center of the bag is 2425C. That is hot enough to melt steel!! If you add a cotton layer it would have little effect on the temperature (R<<1), but you would be dead after a night in your 100 sleeping bags. If I buy better quality R=2 sleeping bags it will be 5,000C at equilibrium.
We will all be dead if we follow your advice to do nothing about Global Warming.
GwsB,
At 547 you said:
"It is based on the idea that photons don't just disappear. They may be absorbed, but are emitted again within a fraction of a second. So I have problems with the first paragraph of post 546 by Michael Sweet which seem to suggest that there is no conservation of photons." my emphasis
This is incorrect. The CO2 molecule has many collisions (millions or higher) with other molecules before it can emit a new photon. The collisions convert the photon's energy into heat in the surrounding molecules.
As soon as a photon is absorbed by a CO2 molecule, the energy of the photon is converted into vibrational energy in the molecule and the photon no longer exists. The collision rate is many orders of magnitude faster than the emission rate so the energy is distributed by collisions to other molecules in the air. Other CO2 molecules, that are boosted into the excited state by molecular collisions, emit photons that effectively replace the original photon.
This is the primary mode of heat transfer in the atmosphere so it cannot be considered negligible.
It is my understanding that little heat is "reflected" back. Energy is absorbed by a layer of the atmosphere. Then new photons are emitted, both up and down, according to the temperature of the layer as described by the Boltzmann equation.
It seems to me that you are trying to model a system you do not understand well. I recommend you read what specialists in the field say to learn faster how the greenhouse effect works.
The emission altitude (about 10,000 meters) is the key point for the greenhouse effect. Here the atmospheric pressure is only about 25 kPa, 1/4 sea level pressure, and the temperature is -50C. The cold freezes out water (vapor pressure of .0039 kPa versus 1.2 kPa at 10C) and the effective CO2 concentration is 1/4 sea level concentration. Saturation is not an issue at 10,000 meters, nor is overlap of water bands. Discussing saturation at the Earth's surface is incorrect.
GwbS@542:
Please do not make strawman arguments. I explicitly said that the diagram in comment 529 applies to the absorption ONLY of radiation, and that I have not considered emission. In comment 534 I give a list of other factors that must be considered. #3 is the mission of radiation. You do yourself no favours by arguing against a position that I have explicitly addressed as incomplete. When ONLY considering absorption, the decay is indeed exponential, and when considering the probabilty of a surface-emitted photon reaching space in one step, absorption is the only relevant factor. Photons emitted in the atmopshere above the surface are - by definition - not emitted from the surface..
You also refer to "reflected" IR radiation. IR radiation is not reflected. Reflection results in photons travelling in a different direction, but remaining at the same wavelength/frequency as they were before reflection. IR radiation is first absorbed, then re-emitted. The emission, as others have stated, is not dependent on the wavelength of the radiation that was absorbed - it depends on the temperature and characteristics of the molecule that is doing the emitting.This may be another CO2 molecule, but it may also be another greenhouse gas. It almost certainly won't be the exact same molecule that did the absorbing. This distinction between "reflection" and "absorption/re-emission" is critical in understanding atmospheric radiation transfer, and you do yourself no favours by conflating the two.
In 547, you state "They may be absorbed, but are emitted again within a fraction of a second". This is basically true, but the amount of time it takes a CO2 molecule to lose the energy by collision is a lot shorter than a "fraction of a second". Eli Rabett has done the math for us:
http://rabett.blogspot.com/2013/04/this-is-where-eli-came-in.html
The time estimate between collisions is 10 us. A CO2 molecule that absorbs IR radiation almost always loses it to other molecules via collision. CO2 molecules that emit IR radiation are almost alwys getting that energy from other collisions.
You also state "the fraction exiting at the top is inversely proportional to the length of the column (or the density)." Physical measurements in units of distance are irrelevant. What matters is the number of particles/molecules/etc. along a path. This varies with altitude depending on the local absolute concentration (not ppm, but molecules/unit volume).
Proper radiation calculations take this into effect.
I repeat what I said in post 534: "The only "saturation" that occurs is for useless and innacurate descriptions of the process." That specific wavelengths show zero direct tranmission of radiation from the surface to space is not an argument against the effects of increasing atmospheric CO2.
PringlesX @ 548:
You say you "found a [sic] interesting site from chicago university that simulates the band saturation."???
How did you find it? That is the exact link I gave you in comment #540. You are reading the comments people make in response to your posts, aren't you?
Now, given that you have provided a series of graphs from that model "that simulates the band saturation", can you please provide us with an explanation of:
Right now, it looks like you are just throwing stuff at the wall hoping something sticks.
The hole tread starts with an even simpler analogy of a water tank with pipes used to try to debunk the saturation effect.
With little googling, i found my analogy is used elsewhere, so it seems i wasnt that far out anyway. (see links)
So i am interested if its possible to debunk the saturation effect by setting up the scenario in the more correct way.
https://www.acs.org/content/acs/en/climatescience/climatesciencenarratives/a-greenhouse-effect-analogy.html
https://skepticalscience.com/SkS_Analogy_09_Greenhouse_effect_stack_of_blankets.html
Bob,
Your link to Eli Rabbit was very informative. I had not previously seen data on how many collisions are needed to relax a CO2 molecule. Previous discussions I have seen suggested 5-10 collisions. Eli Rabbit provided data showing 105 collisions were needed!! That meant that there are only about 100,000 relaxing collisions before the average time of emission and not many millions as I posted above. The rate is a little lower at the escape altitude because it is colder and the concentration of molecules is lower.
The point that most excited CO2 molecules relax and distribute their energy to nearby molecules and do not re-emit the photon still stands.
It seems like i wasnt alone to make that analogy.
If possible, please change the scenario in any way you like, that explains what you believe is happening during a CO2 increase.
https://www.acs.org/content/acs/en/climatescience/climatesciencenarratives/a-greenhouse-effect-analogy.html
https://skepticalscience.com/SkS_Analogy_09_Greenhouse_effect_stack_of_blankets.html
Pringlesx,
It seems to me that your analogy fails becasue you used way too many sleeping bags. (100 bags with doubling CO2 equal to the hundredth bag).
A better analogy would be one sleeping bag with doubling CO2 equal to another bag.
You also use a base concentration of CO2 as 400 ppm. The pre-industrial revolution concentration of CO2 was 270 ppm so it will be doubled at 540 ppm and not 800 ppm as you stated.
Analogies are a tool for promoting understanding by transferring understanding from a known process into a new area where the elements of the analogy are applicable. They are especially useful for explaing things to people who lack the technical background to work through real process.
What you cannot do is disapprove a theory by inappropriate use of an analogy.
If you want to prove some theory is wrong, then you need to show that correct application of the theory results in predictions that are incompatible with observation. Radiative theory so far spectacularly matches observation. You need to focus on understanding rather than looking for some reason to dismiss science.
What you cannot do is disapprove a theory by inappropriate use of an analogy.
How do you feel about that the whole tread starts of with using an analogy of a water tank.
"Lets think about a simple analogy: We have a water tank."
Pringlesx,
It seems to me that your analogy fails becasue you used way too many sleeping bags. (100 bags with doubling CO2 equal to the hundredth bag).
A better analogy would be one sleeping bag with doubling CO2 equal to another bag.
It has been demonstrated that the difference is happening in the TOA. The transmission layer. And the CO2 is saturated in its absorption band. And the athmosphere is not only CO2, GHE is mostly due to water vapour.
So go from one sleeping bag into two sleeping bags is very off.
Scaddenp:
Analogies are a tool for promoting understanding by transferring understanding from a known process into a new area where the elements of the analogy are applicable.
They are especially useful for explaing things to people who lack the technical background to work through real process.
I agree 100%. So is it possible in this case? If you were to have a lecture for a room of people with different backgrounds. What would you say is happening going from 285ppm to 400ppm to 500ppm?
Thanks in advance.
PringlesX @563,
The usual 'adding layers of insulation' analogy only works so far. It is the reduced temperature at the altitude where the IR emits into space that sits at the heart of the AGW mechanism. This is thus not akin to an extra insulating layer which maintains the outside layer temperature and boosts the inner layer temperature with more layers. Your idea of leaky outer insulation @549, or perhaps a space blanked backed by insulation layers, may be a way to a better physical representation in the analogy, but I'm not entirely sure it would greatly assist understanding.
Concerning a 'lecture', it depends if you are just describing the actual GHG mechanism (which would on its own take about 3 minutes to fully explain) or an actual 'lecture' which can be usefully stretched to include background stuff like the S-B relationship, Planck spectrum, depth of the atmosphere, IR path-lengths, outward radiation at the TOA, why GHGs are GHGs, why they operate at particular wavelengths, etc; stuff you are probably already familiar with.
PringlesX - CO2 is not saturated where it matters, at the TOA where emission to space occurs. And we have direct evidence of that, for example Harries et al 2001, which demonstrates that there is decreasing energy leaving the TOA at greenhouse gas absorption frequencies between 1970-1997, creating an energy imbalance between incoming and outgoing radiation that can only result in the entire atmosphere warming.
As to analogies - you can draw parallels between aspects of known and unknown systems with an analogy for instructive purposes, but the analogy isn't the real thing. You cannot disprove with analogies, only with the real science and system in question. In logic this is referred to as the False Analogy fallacy - easy to fall into, but best avoided.
"How do you feel about that the whole tread starts of with using an analogy of a water tank."
There are three level of explanation on this topic. Basic, Intermediate, Advanced. The Basic version starts with an analogy because it is trying to help someone new to the topic, without technical background, understand the issue.
You appear to be trying to disprove established science. Nothing wrong with that - science makes progress that way - but you cannot do that through pushing an analogy. Most break down at some point. You need to start with the Advanced and then move to a textbook on radiative physics if you have a strong reason to believe CO2 is saturated, but I dont think you have grasped the importance of the temperature profile.
Just remember, observations win in science. What we observe matches the theory.
Thr insulation argument is nonsence!
qball17, science has always been political: thats exactly how your mates got rich and powerful in the first place and why you are defending their right to remain so....
" What would you say is happening going from 285ppm to 400ppm to 500ppm?"
Well simply that theory predicts that globally averaged irradiation of the surface will increase by 3.7W/m2 for every doubling of CO2; and that measurements OLR and DLR confirm these calculations.
The model in 542 is wrong I have to admit. It is wrong for two reasons:
1) My impression was that vibrational energy and the kinetic energy mv2/2 were systems with little interaction. That is not the case. Michael Sweet in post 553 and Bob Loblaw in post 554 correct me here. Here is another reference (which gives a proportion of 10**9 instead of 10**5)
https://sealevel.info/Happer_UNC_2014-09-08/Another_question.html
So in the new model the photon at a wavelength of 15 μm travels an average of 25 m before being absorbed by a CO2 molecule which goes into a vibrational state, and which then collides (whatever that means (distance between the centers of the molecules less than the minimum of the two radii?)) with a Nitrogen or Oxygen molecule and falls back into the zero vibrational state (why?) and transfers the vibrational energy into kinetic energy over the two molecules. So CO2 transforms the energy of photons of certain wavelengths into kinetic energy of the atmosphere close (around 25 m) to the position where the photon was emitted.
2) According to Fig 1 in Zhong & Haigh (2013) of the 239 W/m2 outgoing longwave radiation only 22 W/m2 comes directly from the earth. This is from Trenberth & Fasullo (2012). In Tremberth, Fasullo & Kiehl (2009) it is still 40 W/m2.
Looking down from outer space for each photon leaving the earth system at TOA (which is 100 km above the surface according to Google. Is that correct?) one should be able to specify its wavelength and the level above the earth surface where it originated. Around 9% originate at the surface. It would seem that 90% originates close to the surface, say less than 1 or 2 km, except for the wavelengths around 15 mm, which originate at 10 km. (In figure 4 in Zhong and Haigh (2013) the red line follows the Boltzmann-curve for 290K rather than 260K, see figure 3, the temperature at 5 km).
The saturation of CO2 for certain wavelengths shown in the black blue and green graphs in Figure 6(c) in Zhong & Haigh (2013) suggest a transmission which decreases like the inverse of the concentration of CO2 as it approaches the limit value. That agrees with the model in post 542 but I do not see how the new model will give this result.
I think concentration (ppm) is the variable of interest, not density (parts per m3). The twenty layers of my original model each contain the same amount of matter. Their height may vary. The effective CO2 concentration at 10 or 20 km is the same as at sea level. See for instance Aoki et al. (2003) Carbon dioxide variations in the stratosphere over Japan, Scandinavia and Antarctica. Tellus (2003) 55B, 178--186. CO2 is 50% heavier than oxygen or nitrogen, so one would expect it to settle down at the bottom. If it did it would form a layer of pure CO2 more than three meters high. A hundred years ago that was only a bit more than two meters!
You are progressing a little.
Concentration is measured as Molarity which is moles per liter. Ppm is a fraction of particles of dry air and is not concentration . For gases, molarity is directly proportional to pressure. This varies with height. Sometime pressure is used for concentration.
It is a waste of time to attempt to model systems you do not understand. The system is not saturated.
What michael sweet said...
Also, pressure is simply the weight of all overlying gases. If air density were constant, air pressure would drop linearly with height. Air density isn't constant, because pressure drops with height. In the end, air pressure basically decreases logarithmically with height (to a first approximation).
https://en.wikipedia.org/wiki/Atmospheric_pressure
In atmospheric models, they often use pressure as the vertical coordinate. Layers spaced equally in pressure would be roughly equally-spaced on a log(height) scale, or logarithmcially-spaced on a linear height scale.
Three weeks ago there was a congress in Amsterdam of Flat Earthers. I don’t believe the earth is flat. Neither do you. But what do you reply if your child asks “Why isn’t the earth flat?” Do you say “99.9% of the scientists say that the earth is a ball floating in space.” What if your daughter persists, and asks “But why is the earth a ball floating in space?”
I think I can give a sensible answer to that question. If I am asked whether reducing CO2 will have an influence on the climate I am not able to give convincing arguments why a reduction at the present level makes sense.
The Zhong&Haigh (2012) article is well written and convincing, but if one reads it a second and a third time one realizes that the paper contains no arguments. Figure 2 is on a scale which makes it impossible to see the effect of the shoulders and wings of the absorption around 665/cm. The vertical scale covers 12 orders of magnitude. There is no indication of the calculations performed to yield the plots in Figure 6.
Figure 3 suggests that between 750 and 1000/cm the IR radiative flux emitted at TOA follows the Stefan-Boltzmann curve for 280K and between 600 and 750/cm the S-B curve for 220K. This is a model-generated curve but quite close to the observed curve IRIS spectrum dd May 5 1970 over the Sahara at 12.00, see
https://disc.gsfc.nasa.gov/datasets/IRISN4RAD_001/summary
This observation was made almost fifty years ago. Where can one find more recent plots? A clear difference between 1970 and 2019 would be evidence that there is as yet no saturation.
There is a nice explanation of molecular radiation and collisional lifetime (which distinguishes elastic and non-elastic collisions) at https://noconsensus.wordpress.com/2010/08/17/molecular-radiation-and-collisional-lifetime/
DJ Wilson and J Gea-Banacloche (2012) Simple model to estimate the contribution of atmospheric CO2 to the Earth's greenhouse effect (Am. J. Phys. 80, pp 306-315) presents good quantitative arguments for the greenhouse effect of CO2. Does anyone know of more recent literature, in particular on the topic of saturation?
If so that might help me convince my daughter that reduction of CO2 emission makes sense.
Hello,
Most denier arguments i receive i can answer & understand thanks to studies in mechanical engineering and having a decent grasp on the science behind what im reading. But this one has me stumped as its not an area im well versed in, and im wondering if anyone can afford the time to help me understand if what he is saying is correct/incorrect, and why:
___________________
You are linking to Skeptical Science which is under the control of cartoonist John Cook and his friends. This website has been repeatedly known to provide false information and is deceitful. But I will address the issues raised here in the claim that the effect is not saturated.
There is a limited amount of IR emitted from the Earth at each frequency.
At the center of the 15 micron band ALL of the IR is captured by CO2. There can be no more captured. This is not up for debate in any way shape or form.
The method by which IR is captured by CO2 depends upon the ability of the CO2 molecule to match the IR being emitted in both frequency and in orientation of the charge formed by the atoms. The frequency of IR has to match the frequency of the CO2 molecules. It also has to match the orientation in space of the charge of the molecule.
The CO2 molecule vibrates at a certain frequency determined by the atomic weights and charges of the Oxygen and Carbon Atoms. This frequency has a minor variation from the center frequency as one travels outward from the center of the 15 micron band.
SO as more CO2 molecules are added to the atmosphere we see absorption increase at the center of the 15 micron band so that total absorption of all the IR is accomplished closer to the ground. No additional IR becomes absorbed. The Center becomes saturated with respect to its ability to absorb IR. No more IR is absorbed in the center.
Will some CO2 molecules added capture a few additional IR photons further from the center. Yes, But the numbers will be constantly declining in a logarithmic fashion because the CO2 is not vibrating at the correct frequency. This declining logarithmic function is well known because the frequency distribution of the CO2 molecule causes it to not absorb IR at the edges of the 15 micron band. This gives rise to the requirement of a doubling of CO2 per unit increase of temperature. So if for example there is a 1 degree increase for a doubling of CO2 then for the next degree of increase you need 4 times as much CO2 added.
The function of the ability of CO2 to absorb IR follows a bell as it drops off. Only at the Center of the 15 micron band can any absorption of note take place.
The analogy of restricted flow given in the website is totally wrong. You are not adding water which is restricted by the diameter of an outflow pipe. You are adding heat to the atmosphere which has several methods for releasing it from the Earth, As CO2 populations are added there are more molecules in the stratosphere and above to release the energy to space. CO2 does not just stratify near the ground. Further CO2 does not act as a reservoir for IR energy. The energy is spread over all the atmospheric particles s kinetic energy. Oxygen, Nitrogen, H2O are all absorbing the kinetic energy and being transported vertically and toward the poles throughout the atmosphere.
CO2 molecules become re-excited at every level of the atmosphere via collision. 1.4 % in the 15 micron band frequencies. Throughout the atmosphere below the Stratosphere, The re-excitation of these molecules is a net reduction in atmospheric temperature. Eventually there are feedbacks that serve to increase emission to space such as convection, transfer via circulation of Hadley Cells to the poles and the fact that at the poles emission to space takes place at a lower height. If these feedback mechanisms were not present the Earth would have burned to a cinder long ago. But in the lower atmosphere the excited molecules are quenched again and again by collision they cannot add to the temperature because they subtracted from it to become excited once more.
So why is there this idea that CO2 can absorb outside its natural frequency range floating about on Skeptical Science? Because there are experiments performed with CO2 laser systems where the populations within the laser are manipulated artificially and you CAN cause an expansion of the absorption range by applying charges. It does not occur naturally. But you are considered a know nothing by SS and they have no objection to misleading you. within the laser. this does not occur in the natural world.
The explanation of IR escaping from higher colder regions therefore less IR can escape because it is not energetic enough? Well Electromagnetic radiation energy levels are not determined by temperature. They are determined by frequency. Every electronic technician will tell you that a received signal strength depends on frequency. For example to transmit a VHF TV signal requires substantially less current draw than to transmit a UHF TV signal. That is why Channel 6 in Philadelphia refuses to switch to a UHF channel. The range of the signal is lower but it costs OH so much less. If a 15 micron band photon escapes then a 15 micron band photon escapes and the energy levels are the same.
The idea of IR re-radiation is a false one. Because the collisions in the lower atmosphere constantly are quenching an excited CO2 molecule before it can re-emit. The timing constants are on the order of a billion collisions to one re-emission governed by the Einstein A co-efficient. Only in the upper atmosphere where the molecules are far enough apart to allow for there to be no quenching by collision is there any significant re-emission. This is how the Earth loses energy.
The AERI instruments at the Great Plains and North Slope of Alaska detect IR directly. They are designed to detect a range of frequencies and CO2 is one of them.They are unable to detect incoming CO2 IR without manipulating the received signal against a model frequency. The signal is so small that it can be dismissed as an artifact of generating the simulated signal.
The concept of IR scattering is a false one. The photon either passes through the atmosphere or it is absorbed. At the edges of the absorption curve some IR photons radiate directly to space at 186000 miles per second and some is absorbed and converted directly to heat. It depends on whether or not the CO2 molecule is resonant with the photon frequency.
___________________
any assistance is greatly appreciated, just looking to further understand the issues and evidence/counterevidence i may come across
Sgt_Wookie , your friend's comments are an interesting mixture of truths, half-truths, and plain falsehoods.
Somehow, for whatever reason [probably Motivated Reasoning] he has gotten himself into a tangled jungle of confusion. Perhaps he is arguing in bad faith (and is trying to mislead himself and/or his readers) ~ or perhaps it's worse than that, for in places he is bordering on a "word salad" of scientific terms.
He needs to go back to the basic textbooks, and start from scratch. Though I suspect he has too much hubris to accept that the expert scientists have knowledge that he himself lacks. If he were more reasonable, he could start by reading the OP of this thread, and the 500-ish comments thereafter (which also contain some pearls of explanation). But he has closed his mind to the mainstream science (science, easily found via SkS).
Best if you find some indirect way of exposing him. You would waste too much time in correcting him point by point, for he seems the tiresome argumentative sort of fellow who would spend hours in a rearguard battle as he retreats.
(BTW, I love his comment: "[SkS] has been repeatedly known to provide false information and is deceitful." What a laugh! And another strong sign of the Dunning-Kruger Syndrome.)