Is the CO2 effect saturated?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
The notion that the CO2 effect is 'saturated' is based on a misunderstanding of how the greenhouse effect works. |
|||||||
Climate Myth...
CO2 effect is saturated
"Each unit of CO2 you put into the atmosphere has less and less of a warming impact. Once the atmosphere reaches a saturation point, additional input of CO2 will not really have any major impact. It's like putting insulation in your attic. They give a recommended amount and after that you can stack the insulation up to the roof and it's going to have no impact." (Marc Morano, as quoted by Steve Eliot)
At-a-Glance
This myth relies on the use (or in fact misuse) of a particular word – 'saturated'. When someone comes in from a prolonged downpour, they may well exclaim that they are saturated. They cannot imagine being any wetter. That's casual usage, though.
In science, 'saturated' is a strictly-defined term. For example, in a saturated salt solution, no more salt will dissolve, period. But what's that got to do with heat transfer in Earth's atmosphere? Let's take a look.
Heat-trapping by CO2 in the atmosphere happens because it has the ability to absorb and pass on infra-red radiation – it is a 'greenhouse gas'. Infra-red is just one part of the electromagnetic spectrum, divided by physicists into a series of bands. From the low-frequency end of the spectrum upwards, the bands are as follows: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Gamma rays thus have a very high-frequency. They are the highest-energy form of radiation.
As our understanding of the electromagnetic spectrum developed, it was realised that the radiation consists of particles called 'photons', travelling in waves. The term was coined in 1926 by the celebrated physicist Gilbert Lewis (1875-1946). A photon's energy is related to its wavelength. The shorter the wavelength, the higher the energy, so that the very high-energy gamma-rays have the shortest wavelength of the lot.
Sunshine consists mostly of ultraviolet, visible light and infra-red photons. Objects warmed by the sun then re-emit energy photons at infra-red wavelengths. Like other greenhouse gases, CO2 has the ability to absorb infra-red photons. But CO2 is unlike a mop, which has to be wrung out regularly in order for it to continue working. CO2 molecules do not get filled up with infra-red photons. Not only do they emit their own infra-red photons, but also they are constantly colliding with neighbouring molecules in the air. The constant collisions are important. Every time they happen, energy is shared out between the colliding molecules.
Through those emissions and collisions, CO2 molecules constantly warm their surroundings. This goes on all the time and at all levels in the atmosphere. You cannot say, “CO2 is saturated because the surface-emitted IR is rapidly absorbed”, because you need to take into account the whole atmosphere and its constant, ongoing energy-exchange processes. That means taking into account all absorption, all re-emission, all collisions, all heating and cooling and all eventual loss to space, at all levels.
If the amount of radiation lost to space is equal to the amount coming in from the Sun, Earth is said to be in energy balance. But if the strength of the greenhouse effect is increased, the amount of energy escaping falls behind the amount that is incoming. Earth is then said to be in an energy imbalance and the climate heats up. Double the CO2 concentration and you get a few degrees of warming: double it again and you get a few more and on and on it goes. There is no room for complacency here. By the time just one doubling has occurred, the planet would already be unrecognisable. The insulation analogy in the myth is misleading because it over-simplifies what happens in the atmosphere.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
This myth relies on the use of a word – saturated. When we think of saturated in everyday use, the term 'soggy' comes to mind. This is a good example of a word that has one meaning in common parlance but another very specific one when thinking about atmospheric physics. Other such words come to mind too. Absorb and emit are two good examples relevant to this topic and we’ll discuss how they relate to atmospheric processes below.
First things first. The effect of CO2 in the atmosphere is due to its influence on the transport of 'electromagnetic radiation' (EMR). EMR is energy that is moving as x-rays, ultraviolet (UV) light, visible light, infrared (IR) radiation and so on (fig. 1). Radiation is unusual in the sense that it contains energy but it is also always moving, at the speed of light, so it is also a form of transport. Radiation is also unusual in that it has properties of particles but also travels with the properties of waves, so we talk about its wavelength.
The particles making up radiation are known as photons. Each photon contains a specific amount of energy, and that is related to its wavelength. High energy photons have short wavelengths, and low energy photons have longer wavelengths. In climate, we are interested in two main radiation categories - firstly the visible light plus UV and minor IR that together make up sunshine, and secondly the IR from the earth-atmosphere system.
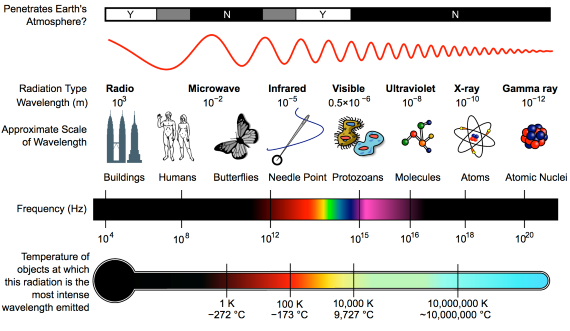
Fig. 1: diagram showing the full electromagnetic spectrum and its properties of the different bands. Image: CC BY-SA 3.0 from Wikimedia.
CO2 has the ability to absorb IR photons – it is a 'greenhouse gas'.So what does “absorb” mean, when talking about radiation? We are all familiar with using a sponge to mop up a water spill. The sponge will only absorb so much and will not absorb any more unless it's wrung out. In everyday language it may be described, without measurements, as 'saturated'. In this household example, 'absorb' basically means 'soak up' and 'saturated' simply means 'full to capacity'. Scientific terms are, in contrast, strictly defined.
Now let's look at the atmosphere. The greenhouse effect works like this: energy arrives from the sun in the form of visible light and ultraviolet radiation. A proportion reaches and warms Earth's surface. Earth then emits the energy in the form of photons of IR radiation.
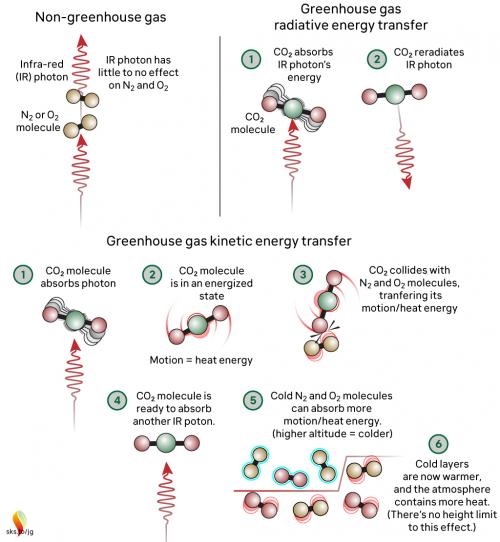
Greenhouse gases in the atmosphere, such as CO2 molecules, absorb some of this IR radiation, then re-emit it in all directions - including back to Earth's surface. The CO2 molecule does not fill up with IR photons, running out of space for any more. Instead, the CO2 molecule absorbs the energy from the IR photon and the photon ceases to be. The CO2 molecule now contains more energy, but that is transient since the molecule emits its own IR photons. Not only that: it's constantly colliding with other molecules such as N2 and O2 in the surrounding air. In those collisions, that excess energy is shared with them. This energy-sharing causes the nearby air to heat up (fig. 2).
Fig. 2: The greenhouse effect in action, showing the interactions between molecules. The interactions happen at all levels of the atmosphere and are constantly ongoing. Graphic: jg.
The capacity for CO2 to absorb photons is almost limitless. The CO2 molecule can also receive energy from collisions with other molecules, and it can lose energy by emitting IR radiation. When a photon is emitted, we’re not bringing a photon out of storage - we are bringing energy out of storage and turning it into a photon, travelling away at the speed of light. So CO2 is constantly absorbing IR radiation, constantly emitting IR radiation and constantly sharing energy with the surrounding air molecules. To understand the role of CO2, we need to consider all these forms of energy storage and transport.
So, where does 'saturation' get used in climate change contrarianism? The most common way they try to frame things is to claim that IR emitted from the surface, in the wavelengths where CO2 absorbs, is all absorbed fairly close to the surface. Therefore, the story continues, adding more CO2 can’t make any more difference. This is inaccurate through omission, because either innocently or deliberately, it ignores the rest of the picture, where energy is constantly being exchanged with other molecules by collisions and CO2 is constantly emitting IR radiation. This means that there is always IR radiation being emitted upwards by CO2 at all levels in the atmosphere. It might not have originated from the surface, but IR radiation is still present in the wavelengths that CO2 absorbs and emits. When emitted in the upper atmosphere, it can and will be lost to space.
When you include all the energy transfers related to the CO2 absorption of IR radiation – the transfer to other molecules, the emission, and both the upward and downward energy fluxes at all altitudes - then we find that adding CO2 to our current atmosphere acts to inhibit the transfer of radiative energy throughout that atmosphere and, ultimately, into space. This will lead to additional warming until the amount of energy being lost to space matches what is being received. This is precisely what is happening.
The myth reproduced at the top – incorrectly stating an analogy with roof insulation in that each unit has less of an effect - is misleading. Doubling CO2 from 280 ppm to 560 ppm will cause a few degrees of warming. Doubling again (560 to 1130 ppm) will cause a similar amount of additional warming, and so on. Many doublings later there may be a point where adding more CO2 has little effect, but recent work has cast serious doubt on that (He et al. 2023). But we are a long, long way from reaching that point and in any case we do not want to go anywhere near it! One doubling will be serious enough.
Finally, directly observing the specific, global radiative forcing caused by well-mixed greenhouse gases has - to date - proven elusive. This is because of irregular, uncalibrated or limited areal measurements. But very recently, results have been published regarding the deep reinterrogation of years of data (2003-2021) from the Atmospheric Infrared Sounder (AIRS) instrument on NASA's Aqua Satellite (Raghuraman et al. 2023). The work may well have finally cracked the long-standing issue of how to make finely detailed, consistent wavelength-specific measurements of outgoing long-wave radiation from Earth into space. As such, it has opened the way to direct monitoring of the radiative impact (i.e. forcing + feedback) of greenhouse gas concentration changes, thereby complimenting the Keeling Curve - the longstanding dataset of measured CO2 concentrations, down at the planet's surface.
Note: Several people in addition to John Mason were involved with updating this basic level rebuttal, namely Bob Loblaw, Ken Rice and John Garrett (jg).
Last updated on 31 December 2023 by John Mason. View Archives































 Arguments
Arguments





































Eclectic @750
Well, it is ultimately the sun that drives warming of the entire earth, both the solid part and the atmosphere. The primary mechanism for the sun warming the earth is the absorption of visible light (from the sun) into the solid portion of the earth which then acts as a near blackbody. This blackbody radiator then warms the atmosphere by conduction and convection.
It should be noted that convection is important for the CO2 greenhouse effect to work since the 15 micron absorption band of CO2 is strong enough to pack the thermal radiation from the entire band into a layer at the surface just a few tens of meters thick. Without convection, this would give us a very hot surface. Also, however, there would be a steep temperature gradient near the surface which would result in a strong pressure gradient. This pressure gradient would then drive updrafts that would carry excess heat away from the surface. In this manner, convection stops excessive heating of the bottom layer of the atmosphere.
Finally, I am not familiar with Trenberth's summary. Could you give me a link or a reference?
CallItAsItIs @751 & prior :-
Yes indeed, it is "ultimately the sun that drives warming of the entire earth, both the solid part and the atmosphere".
And for practical purposes, we can ignore the (fission) heat generated subterraneously, and heat generated by tidal motion, and heat generated by combustion of fossil fuels etcetera.
But when discussing atmospheric physics, it would be substantially wrong to ignore the direct heating of the atmosphere by sunlight (as short-wave radiation, including UV radiation). There is a famous "cartoon" by Dr Trenberth, giving a summary of the various energy flows in the terrestrial atmosphere ~ quite colorful, though the exact figures utilized are perhaps only accurate to about +/- 1%.
Forgive me, but I feel you are pulling my leg, when you say (with your atmospheric interest) that you have not heard of Trenberth. Just like I would feel when a Rocket Scientist says he has not heard of Newton.
CallItAsItIs @ 749:
Yes, we need to get some basics straight. You say:
...and at this point, you have the basics horribly, horribly wrong. Energy conservation applies to all forms of energy. There is no "energy conservation" that applies solely to IR radiation. Energy is conserved in a system that obtains all energy as solar radiation, and emits the same amount of energy solely as IR radiation. When you isolate one form or another, there is absolutely no requirement that solar energy be conserved, or IR energy be conserved.
From this basic misunderstanding on your part, you have created a cartoon physics that bears no resemblance to reality.
You then continue with:
This, frankly, is "not even wrong". Try reading Wikipedia's page on thermal radiation. The opening paragraph starts with:
Since you can't even get the basics right, the rest of your argument is completely illogical.
As for Eclectic's reference to the Trenberth energy diagram, here it is. Note that the only "conservation of energy" rules that applies is to the entire diagram as a whole - not the individual components (IR, solar, etc.)
...and to respond to CallItAsItIs @ 751, where he says:
This, and the rest of your comment at 751, is essentially correct. Yet for some strange reason, you completely ignore all this atmospheric heating by conduction and convection when you claim that there is no possible source of energy to drive emission of 15um radiation within the atmosphere. In comment 741 (responding to my comment 732, where I said the atmosphere is a source of IR radiation), you stated:
So, which is it? Does conduction and convection add energy to the atmosphere? Energy that is then available to be emitted as IR radiation? Or is it solely the input of IR radiation that can provide a source of energy for the emission of IR radiation?
The inconsistency of your arguments is astounding.
As a followup to my comment at 753, I grabbed the Trenberth diagram from this SkS post, where it is figure 6. The caption for that figure gives the source as this.
CallItAsItIs @748,
Since you ask a specific question: Kirchhoff’s Law is absorptance = emittance (at thermal equilibrium). It works like this: photon is absorbed by CO2. CO2 molecules collide with N2 and O2 to come to thermal equilibrium (i.e., same temperature). CO2 molecule emits photon. The net effect at equilibrium is a pass-through of energy unless there is a change that upsets equilibrium.
You emphasize that the 15-micron absorption band (by which I think you mean the approximately 14-16 micron band and not a few 14.9 micron peak lines) absorbs completely within 10 meters of the surface. @725 you say “CO2 can still emit IR radiation (at any wavelength) but can no longer absorb within this band.” @751 the 15 micron absorption band of CO2 is strong enough to pack the thermal radiation from the entire band into a layer at the surface just a few tens of meters thick.” On the other hand, @740 you seem to acknowledge that the spectra showing upward IR within the band are correct, not just at 70 km but also at lower altitudes. If you were consistent, there would be zero intensity for the band because it would have all been absorbed at low altitude. Maybe I give you too much credit there, since your interpretation of the spectra was not correct and you said CO2 can still emit but no longer absorb, so maybe you were not being inconsistent but just do not apply Kirchhoff's Law.
Clarification: Pass-through of photons is more descriptive than pass-through of energy. Photons don't have wavelength so it risks mixing up the concept of electromagnetic radiation as waves or particles. However, they are emitted at a specific frequency so a stream of photons can be described as wavelength. A column of the atmosphere has conservation of energy, not conservation of photons. Radiant energy follows the temperature profile of the atmosphere. Stefan-Boltzmann Law: Intensity = emittance (specific to wavelength) x Stefan Boltzmann constant x absolute temperature to the 4th power.
Charlie Brown @ 757:
Minor correction - yes, individual photons have the properties of both wavelength and frequency. Part of the weirdness of the particle/wave duality of light/EM radiation.
The wavelength, frequency, and energy of a photon are all tied together. If it is a 15um photon, that also sets its frequency and energy level. All 15um photons are the same.
A "stream" of photons is not like a stream of water where everything is connected. Even a stream of photons is just a bunch of individual photons passing one at a time past a point in space. When silicon diodes are used as radiation sensors, they are basically "photon counters".
When a higher temperature means more energy emitted at a specific wavelength, it just means "more photons", not "more energy in each photon".
Oh, and when CO2 (or anything else) "absorbs a photon", it does not store the photon, it just stores the energy. The photon ceases to be. It is no more. It is a "late" photon.
When CO2 (or anything else) emits a photon, it does not get one out of storage. It creates one using energy stored elsewhere in the molecule.
Energy is conserved, but there is no such law as "conservation of photons", as Charlie Brown has already stated in comment 757.
Charlie_Brown @756
It works like this: photon is absorbed by CO2. CO2 molecules collide with N2 and O2 to come to thermal equilibrium (i.e., same temperature). CO2 molecule emits photon. The net effect at equilibrium is a pass-through of energy unless there is a change that upsets equilibrium.
Wrong! It works like this: Photon is absorbed by CO2. Energized CO2 molecule collides with N2 or O2. In this collision, CO2 molecule releases the energy it gained from the photon absorption which then becomes kinetic energy of both molecules, thereby raising temperature. At the same time, of course, the CO2 molecule returns to it original state, ready to absorb another photon.
In your explanation, warming of any of the atmospheric gases would not be possible with violating energy conservation.
Bob Loblaw @754
Convection and conduction are mechanisms for transferring energy. They are not sources of energy.
CallitAiItIs @ 760:
Once again, you fail to look at the entire system, and fail to understand what is being said to you.
What Charlie Brown says in 756 is correct. Indeed, his statement that "CO2 molecules collide with N2 and O2 to come to thermal equilibrium (i.e., same temperature)" means exactly the same thing as your statement "CO2 molecule releases the energy it gained from the photon absorption which then becomes kinetic energy of both molecules, thereby raising temperature. "
The catch is that all CO2 molecules are continually emitting radiation. This represents an energy loss. And they make up for that energy loss by colliding with other molecules (such as N2 and O2) and gaining energy when those molecules have higher energy.
The energy transfer via collision between CO2 and other molecules works in both directions, depending on the relative energy level of the individual molecules. (You can't talk about temperature of an individual molecule, as "temperature" is a bulk property of many molecules. Average kinetic energy.)
I have previously pointed you to this blog post at Eli Rabett's, where this is explained well.
Please read some of these additional links you have been pointed to. You continue to embarrass yourself by making elementary errors in physics. Until you unlearn your misconceptions, it is very difficult for you to get an accurate understanding of this subject.
CallItAsItIs @ 761:
You say:
And that is why the Trenberth diagram uses the title "Global Energy Flows".
It may be news to you, but radiation is also a mechanism for transferring energy. Have you never noticed that radiation travels from one place to another? It does so fairly quickly - as a rough approximation, at the speed of light.
CallItAsItIs:
Since you are really big on making sure we agree on basics and terminology, could you please provide us with your definition of "sources of energy"?
Try to remember the principle of conservation of energy while you are at it.
Bob Loblaw @764
Come on, Bob! Learn some physics!
What we call sources of energy depends on our system and what we are trying to determine. In the case of the greenhouse effect, we are trying to determine the warming of the atmosphere due to GHGs tapping energy from the terrestrial IR radiation rising from the surface. This means that the upwelling terrestrial IR radiation is the source. The GHGs catch energy in the form of photons from this source, and convert it to kinetic energy of the atmospheric gases (including the GHGs). These GHGs, however, are not sources since they contribute no energy of their own.
The sun also is a source of energy since it puts out IR radiation which is absorbed by the GHGs and converted into thermal energy in the same manner as the terrestrial IR radiation. The atmosphere's primary source of thermal energy, however, is the upwelling terrestrial radiation since IR radiation is more at the "tail-end" of the solar spectrum.
If we are interested in determining the temperature of the "solid" subterranean earth, then the sun becomes our primary source. In this case, the earth absorbs the EMR from the sun which is mostly in the visible spectrum since that is where the peak solar emissions occur. Also, the atmosphere is transparent to visible EMR (ie. light). The down-welling terrestrail radiation from the atmosphere is another a source, but a much weaker one.
Now that we have (hopefully) gotten it straight as to what is meant by sources of energy, let's get back to the problem-at-hand of assessing saturation of the 15 micron absorption band of CO2. In this case, our source of energy is the upwelling terrestrial radiation within this absorption band. Since the contributions to the total upwelling EMR at different frequencies involve different photons, conservation of energy must hold for each frequency independently of the others. This enables us to use the Beer Lambert law to evaluate the attenuation of each frequency component of the upward-bound IR. And, as indicated in previous posts, intensity contributions within the 15 micron band become pretty miniscule at altitudes well below the TOA.
Bob Loblaw @762
The catch is that all CO2 molecules are continually emitting radiation. This represents an energy loss. And they make up for that energy loss by colliding with other molecules (such as N2 and O2) and gaining energy when those molecules have higher energy.
And how do they get this extra energy? — from Maxwell's Demons! (LOL)
CallItAsItIs @765 ; @766 :-
Each of your "explanations" generates the need for more explanations.
e.g. ~ Why must conservation of energy "hold for each frequency independently of the others" ? [unquote]
[ Even the famous scientific pioneers of the 19th century would be scratching their heads over this novel idea of yours. ]
And your Demonic suggestion that seems to imply that atmospheric molecules cannot (in bulk) gain energy from neighbouring molecules . . . is another novel idea that requires your explanation.
And all this time, you have avoided the IR role (and other roles) of H2O molecules.
And you have not explained why your idea of "TOA" is so very different from that of the mainstream atmospheric physicists.
Yessir, there's much for you to explain ~ to readers and to yourself.
CallItAsItIs - if you look at Trenberth diagram you will notice incoming energy flux at earth surface is 161+333 W/m2 (of course balanced by the same outflow), whereas incoming energy flux at TOA is only 341W/m2. These of course are measured values. How does your unconvential view of physics account for this?
Eclectic @767
Why must conservation of energy "hold for each frequency independently of the others" ?
Because when we break down the EMR into the sum of contributions from the different frequencies it contain, we find that each such contribution is incoherent relative to the others. This means that the energy flux of the entire distribution is simply the sum of the energy fluxes from each contribution. Now, if this is unclear to you, please understand that I cannot pack an entire radiometry textbook into this comment space.
And your Demonic suggestion that seems to imply that atmospheric molecules cannot (in bulk) gain energy from neighbouring molecules . . . is another novel idea that requires your explanation.
No, that idea is not so novel since the occurrence you describe would violate the second law of thermodynamics.
And all this time, you have avoided the IR role (and other roles) of H2O molecules.
That's right since I am only addressing the issue of CO2 band saturation and not H2O greenhouse warming which is completely different.
scaddenp @768
... - if you look at Trenberth diagram you will notice incoming energy flux at earth surface is 161+333 W/m2 (of course balanced by the same outflow), whereas incoming energy flux at TOA is only 341W/m2. These of course are measured values. How does your unconvential view of physics account for this?
I do not address energy balance issues other than those within the atmosphere which apply to the CO2 band saturation issue. I assume a given (but adjustable) intensity value for the 15 micron absorption band at the surface, and see how this radiation is attenuated with respect to altitude. By tuning this intensity value and possibly the absorption factor, we should (hopefully) be able to obtain a balanced energy solution that is also radiometrically correct. From what I have found so far, however, I believe it would be quite at "stretch" to avoid band saturation since we CO2 is such a strong absorber at 15 microns.
CallItAsItIs @770 :-
If I may answer on Scaddenp's behalf ~ I would point out that what you say could have relevance on Mars with a pure CO2 atmosphere (disregarding a trace of nitrogen & argon). But on planet Earth, your explanation clearly fails the reality test.
*
CallItAsItIs @769 :-
Well, I give you points for being entertaining !
My cynical streak impels me to the suspicion that you are using AI-generated language to produce a garbled incoherence of "explanations".
Even better is your novel suggestion that atmospheric molecules cannot give or receive energy to/from other atmospheric molecules (why ~ because Second Law of T , of course, eh what, eh ).
And surely only an AI would assert that H2O molecules have no indirect or direct effect or interaction (kinetic or photonic) with nearby N2 , O2 , or CO2 molecules.
Vraiment une tour de force, mon ami. (Does the AI tackle French, as well? )
CallitItAsItIs @ 765 (where he responds to my request for his definition of "sources of energy"):
You are in no position to tell other people to "learn some physics". Let's start with one of your statements:
Once again, you are wrong. Let's look at Trenberth's diagram again:
You clearly have no idea what this diagram shows. I will point specifically to two arrows in the middle of the diagram, originating at the surface. The ones labelled "Thermals" and "Evapotranspiration". Those are flows of energy from the surface ("source") to the atmosphere (sink, if you like). IR radiation (labelled "Surface radiation") is to the right, and it is not the only transfer of energy from the surface to the atmosphere.
You continue with:
Once again, you ignore anything other than IR radiation. A lot of the sun's direct warming of the atmosphere comes from absorbing non-IR radiation - visible light, and UV radiation. In fact, the main reason that the stratosphere is much warmer than the troposphere is because of UV absorption by ozone. The atmosphere is not completely transparent to visible or UV radiation.
Then you state (with respect to surface heating):
Look at the Trenberth diagram again. Solar radiation absorbed by the surface is 161 W/m2. (On the left side.) If you look on the right side, you see that "Back Radiation" (IR from the atmosphere to the surface) is 333 W/m2. I challenge you to find one reputable source that says 333 is "much weaker" than 161.
..and if you look closely at the IR radiation flows between the surface and the atmosphere (on the right of the diagram), you will see that the net exchange is only +23 W/m2 - the atmosphere only absorbs 356 W/m2 of the 396 W/m2 coming off the surface, but sends 333 W/m2 back to the surface. Contrast that with the 97 W/m2 (17+80) transferred from the surface to the atmosphere by thermals and evapotranspiration, and add in the 78 W/m2 of solar radiation absorbed directly by the atmosphere (in the middle of the diagram) and you get a total of 175 W/m2 of energy added to the atmosphere from sources that are not surface emission of IR radiation.
And then in your closing paragraph, you state (emphasis added):
And this is probably the root cause of your confusion. No, conservation of energy is not something that must hold for each frequency independent of others.
Once CO2 (or any other material) absorbs a photon, the energy gets transformed into another form (thermal/kinetic, chemical, etc.) and the CO2 is free to do whatever it wants to (restricted by physics and chemistry, of course) with that energy. It can emit it as radiation in any frequency of the many it is capable of absorbing or emitting. It can keep it as kinetic (thermal) energy. It can dump it off as kinetic energy to other molecules it collides with as it bounces around in the sky.
The energy contained within the CO2 molecule has no memory of where it came from. Absorption of radiation, kinetic transfer from colliding with other molecules, etc. It's all just energy once it is stored in the molecular structure of the CO2.
Energy conservation only applies to the system as a whole. Your version of "physics" is bordering on crackpot territory.
CallIiAsItIs @ 766:
I need to quote this in its entirety for context. Your comment says:
I assume that you mean "other molecules" when you say "they'. Well, if you read my comment at 772 (and all of my comment at 762) and look closely at the Trenberth diagram, you will discover that the other molecules can get that energy from absorbing IR radiation, visible light, UV radiation, thermal transfers from the surface, evaporation from the surface, or simply by colliding with yet more molecules that have received energy from any of those sources.
Unfortunately, I don't think that any of that is going to sink in for you, since it becoming abundantly clear that you have an extremely strong Morton's Demon filtering your "knowledge" of physics.
One more. CallItAsItIs says in comment 769:
The only things that is incoherent is CallItAsItIs's physics. Absorption and emission of radiation are independent events. Once again, I beg that CallItAsItIs read Eli Rabbet's blog post on the time scales involved in absorption, emission, and collisions with other molecules. [CallItAsItIs: the previous sentence includes the link to that blog post.]
To start, here is the opening section of Eli's post:
I will leave it as an exercise for the reader to decide whether CallItAsItIs falls into class 1, class 2, or both.
One more "one more". CallItAsItIs states in 769:
I challenge you to actually name just one "radiometry textbook" that you have read. Bonus points if you can point to a section of such a book that supports any of your postings here.