Is the CO2 effect saturated?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
The notion that the CO2 effect is 'saturated' is based on a misunderstanding of how the greenhouse effect works. |
|||||||
Climate Myth...
CO2 effect is saturated
"Each unit of CO2 you put into the atmosphere has less and less of a warming impact. Once the atmosphere reaches a saturation point, additional input of CO2 will not really have any major impact. It's like putting insulation in your attic. They give a recommended amount and after that you can stack the insulation up to the roof and it's going to have no impact." (Marc Morano, as quoted by Steve Eliot)
At-a-Glance
This myth relies on the use (or in fact misuse) of a particular word – 'saturated'. When someone comes in from a prolonged downpour, they may well exclaim that they are saturated. They cannot imagine being any wetter. That's casual usage, though.
In science, 'saturated' is a strictly-defined term. For example, in a saturated salt solution, no more salt will dissolve, period. But what's that got to do with heat transfer in Earth's atmosphere? Let's take a look.
Heat-trapping by CO2 in the atmosphere happens because it has the ability to absorb and pass on infra-red radiation – it is a 'greenhouse gas'. Infra-red is just one part of the electromagnetic spectrum, divided by physicists into a series of bands. From the low-frequency end of the spectrum upwards, the bands are as follows: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Gamma rays thus have a very high-frequency. They are the highest-energy form of radiation.
As our understanding of the electromagnetic spectrum developed, it was realised that the radiation consists of particles called 'photons', travelling in waves. The term was coined in 1926 by the celebrated physicist Gilbert Lewis (1875-1946). A photon's energy is related to its wavelength. The shorter the wavelength, the higher the energy, so that the very high-energy gamma-rays have the shortest wavelength of the lot.
Sunshine consists mostly of ultraviolet, visible light and infra-red photons. Objects warmed by the sun then re-emit energy photons at infra-red wavelengths. Like other greenhouse gases, CO2 has the ability to absorb infra-red photons. But CO2 is unlike a mop, which has to be wrung out regularly in order for it to continue working. CO2 molecules do not get filled up with infra-red photons. Not only do they emit their own infra-red photons, but also they are constantly colliding with neighbouring molecules in the air. The constant collisions are important. Every time they happen, energy is shared out between the colliding molecules.
Through those emissions and collisions, CO2 molecules constantly warm their surroundings. This goes on all the time and at all levels in the atmosphere. You cannot say, “CO2 is saturated because the surface-emitted IR is rapidly absorbed”, because you need to take into account the whole atmosphere and its constant, ongoing energy-exchange processes. That means taking into account all absorption, all re-emission, all collisions, all heating and cooling and all eventual loss to space, at all levels.
If the amount of radiation lost to space is equal to the amount coming in from the Sun, Earth is said to be in energy balance. But if the strength of the greenhouse effect is increased, the amount of energy escaping falls behind the amount that is incoming. Earth is then said to be in an energy imbalance and the climate heats up. Double the CO2 concentration and you get a few degrees of warming: double it again and you get a few more and on and on it goes. There is no room for complacency here. By the time just one doubling has occurred, the planet would already be unrecognisable. The insulation analogy in the myth is misleading because it over-simplifies what happens in the atmosphere.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
This myth relies on the use of a word – saturated. When we think of saturated in everyday use, the term 'soggy' comes to mind. This is a good example of a word that has one meaning in common parlance but another very specific one when thinking about atmospheric physics. Other such words come to mind too. Absorb and emit are two good examples relevant to this topic and we’ll discuss how they relate to atmospheric processes below.
First things first. The effect of CO2 in the atmosphere is due to its influence on the transport of 'electromagnetic radiation' (EMR). EMR is energy that is moving as x-rays, ultraviolet (UV) light, visible light, infrared (IR) radiation and so on (fig. 1). Radiation is unusual in the sense that it contains energy but it is also always moving, at the speed of light, so it is also a form of transport. Radiation is also unusual in that it has properties of particles but also travels with the properties of waves, so we talk about its wavelength.
The particles making up radiation are known as photons. Each photon contains a specific amount of energy, and that is related to its wavelength. High energy photons have short wavelengths, and low energy photons have longer wavelengths. In climate, we are interested in two main radiation categories - firstly the visible light plus UV and minor IR that together make up sunshine, and secondly the IR from the earth-atmosphere system.
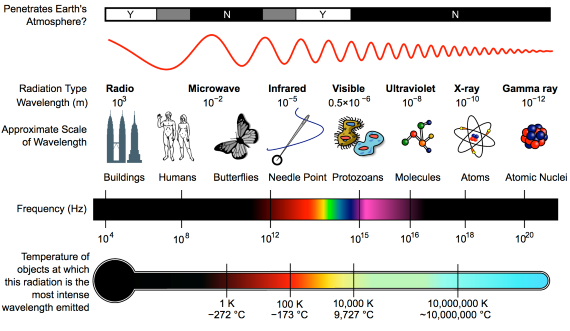
Fig. 1: diagram showing the full electromagnetic spectrum and its properties of the different bands. Image: CC BY-SA 3.0 from Wikimedia.
CO2 has the ability to absorb IR photons – it is a 'greenhouse gas'.So what does “absorb” mean, when talking about radiation? We are all familiar with using a sponge to mop up a water spill. The sponge will only absorb so much and will not absorb any more unless it's wrung out. In everyday language it may be described, without measurements, as 'saturated'. In this household example, 'absorb' basically means 'soak up' and 'saturated' simply means 'full to capacity'. Scientific terms are, in contrast, strictly defined.
Now let's look at the atmosphere. The greenhouse effect works like this: energy arrives from the sun in the form of visible light and ultraviolet radiation. A proportion reaches and warms Earth's surface. Earth then emits the energy in the form of photons of IR radiation.
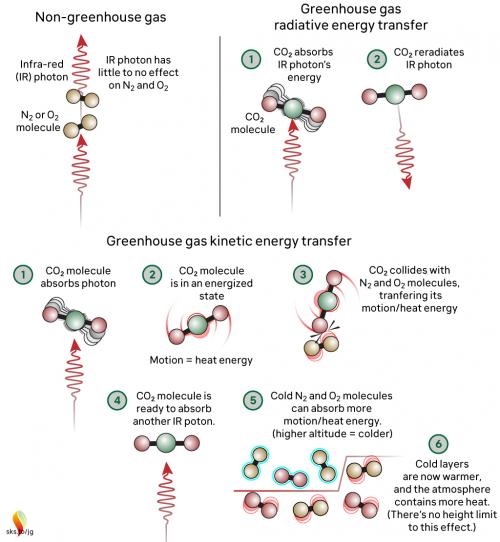
Greenhouse gases in the atmosphere, such as CO2 molecules, absorb some of this IR radiation, then re-emit it in all directions - including back to Earth's surface. The CO2 molecule does not fill up with IR photons, running out of space for any more. Instead, the CO2 molecule absorbs the energy from the IR photon and the photon ceases to be. The CO2 molecule now contains more energy, but that is transient since the molecule emits its own IR photons. Not only that: it's constantly colliding with other molecules such as N2 and O2 in the surrounding air. In those collisions, that excess energy is shared with them. This energy-sharing causes the nearby air to heat up (fig. 2).
Fig. 2: The greenhouse effect in action, showing the interactions between molecules. The interactions happen at all levels of the atmosphere and are constantly ongoing. Graphic: jg.
The capacity for CO2 to absorb photons is almost limitless. The CO2 molecule can also receive energy from collisions with other molecules, and it can lose energy by emitting IR radiation. When a photon is emitted, we’re not bringing a photon out of storage - we are bringing energy out of storage and turning it into a photon, travelling away at the speed of light. So CO2 is constantly absorbing IR radiation, constantly emitting IR radiation and constantly sharing energy with the surrounding air molecules. To understand the role of CO2, we need to consider all these forms of energy storage and transport.
So, where does 'saturation' get used in climate change contrarianism? The most common way they try to frame things is to claim that IR emitted from the surface, in the wavelengths where CO2 absorbs, is all absorbed fairly close to the surface. Therefore, the story continues, adding more CO2 can’t make any more difference. This is inaccurate through omission, because either innocently or deliberately, it ignores the rest of the picture, where energy is constantly being exchanged with other molecules by collisions and CO2 is constantly emitting IR radiation. This means that there is always IR radiation being emitted upwards by CO2 at all levels in the atmosphere. It might not have originated from the surface, but IR radiation is still present in the wavelengths that CO2 absorbs and emits. When emitted in the upper atmosphere, it can and will be lost to space.
When you include all the energy transfers related to the CO2 absorption of IR radiation – the transfer to other molecules, the emission, and both the upward and downward energy fluxes at all altitudes - then we find that adding CO2 to our current atmosphere acts to inhibit the transfer of radiative energy throughout that atmosphere and, ultimately, into space. This will lead to additional warming until the amount of energy being lost to space matches what is being received. This is precisely what is happening.
The myth reproduced at the top – incorrectly stating an analogy with roof insulation in that each unit has less of an effect - is misleading. Doubling CO2 from 280 ppm to 560 ppm will cause a few degrees of warming. Doubling again (560 to 1130 ppm) will cause a similar amount of additional warming, and so on. Many doublings later there may be a point where adding more CO2 has little effect, but recent work has cast serious doubt on that (He et al. 2023). But we are a long, long way from reaching that point and in any case we do not want to go anywhere near it! One doubling will be serious enough.
Finally, directly observing the specific, global radiative forcing caused by well-mixed greenhouse gases has - to date - proven elusive. This is because of irregular, uncalibrated or limited areal measurements. But very recently, results have been published regarding the deep reinterrogation of years of data (2003-2021) from the Atmospheric Infrared Sounder (AIRS) instrument on NASA's Aqua Satellite (Raghuraman et al. 2023). The work may well have finally cracked the long-standing issue of how to make finely detailed, consistent wavelength-specific measurements of outgoing long-wave radiation from Earth into space. As such, it has opened the way to direct monitoring of the radiative impact (i.e. forcing + feedback) of greenhouse gas concentration changes, thereby complimenting the Keeling Curve - the longstanding dataset of measured CO2 concentrations, down at the planet's surface.
Note: Several people in addition to John Mason were involved with updating this basic level rebuttal, namely Bob Loblaw, Ken Rice and John Garrett (jg).
Last updated on 31 December 2023 by John Mason. View Archives































 Arguments
Arguments





































Bob Loblaw @797,
Commenter CallItAsItIs @800 continues to demonstrate a schoolyard approach to this subject which is not appropriate to its scientific nature. If "one could probably show" something then why shouldn't 'one'. Oh, this is because "typesetting equations tends to be a long, grueling task" for him and "most likely" these equations would then be subject to rebuttal "over statements I did not make or that you misunderstood." I think I'd prefer "the dog ate my homework!!"
While the commenter CallItAsItIs appears a lost cause and too far up his own nonsense to see any of his multivarious misconceptions, it would be correct here to ask him to explain his comment @796 and show where exactly it is within Schwarzschild's equation there is "an exponential term that vanishes at high altitudes" and demonstrate from that how it is this would obtain "the exact same result (he has) been claiming through all the ridicule."
Charlie_Brown @799
1) He keeps referring to a single 15-micron band.
Yes — It is common practice to approximate the entire absorption band from 14-16 microns as a band at wavelength 15 microns for the purpose of analyzing the CO2 greenhouse effect, and until now, no one has objected. So, why is it suddenly and issue when I do likewise?
2) Once absorbed, near the surface or anywhere in the atmosphere, Kirchhoff’s Law applies, absorptance = emittance, ...
It seems that you forgot something that you yourself included in comment 756, namely the at equilibrium part, and an atmosphere that is warming is not at equilibrium.
There is more to CallItAsItIs’ misunderstandings, e.g., “Beer's Law is a linear equation.” No, it is exponential.
I will acknowledge some confusion on my part when I made this statement. At that time, I was thinking that Beer's Law was the first order linear differential equation that we solve to get exponential dependence of the spectral intensity on altitude. Anyway, that statement did not affect any of my future arguments.
Finally, lets consider your statement from comment 756
It works like this: photon is absorbed by CO2. CO2 molecules collide with N2 and O2 to come to thermal equilibrium (i.e., same temperature). CO2 molecule emits photon. The net effect at equilibrium is a pass-through of energy unless there is a change that upsets equilibrium.
From what you describe here, it seems to me that we end up with exactly the same system from which we started. Namely, we started with a CO2 molecule and a photon in thermal equilibrium, and in the end, we got the same CO2 molecule in the same state with a similar photon, all in thermal equilibrium. So how is it that we get any warming?
That's all for now, but I may have some more feedback when I have a chance to review your final paragraph some more.
CallItAsItIs @802 :
Just to pick out one of your many errors :-
In your penultimate paragraph you say: "Namely we start with a CO2 molecule and a photon ... and in the end ... all in thermal equilibrium. So how is it that we get any warming?"
That comment of yours demonstrates your gross failure to understand the warming result from the so-called GreenHouse Effect. And your failure to educate yourself at the many sources available on-line.
You have looked at a single "tree", a single cubic meter of air ~ and you turn a blind eye to the fact that the atmosphere becomes progressively thinner with altitude and progressively cooler with altitude (the temperature Lapse Rate of the troposphere, which provides the bulk of the GHE ).
The question for your own introspection is: Why would anyone [such as yourself] choose to ignore the many facts (including densities and lapse rate) that show the mechanism of GHE by CO2 , H2O etcetera? Why would that person [such as yourself] choose to be so un-scientific? ~ is the answer Motivated Reasoning, and/or some other embarrassing condition of the human brain?
Look inwards, CallItAsItIs.
As the sage said: "Know Thyself".
.
CallItAsItIs continues to provide assertions with no evidence. And he is also saying "it's too hard!" when asked to show his math, and shouting "Wrong!" at anyone that points out his misunderstandings.
MA Rodger is correct in comment 801, when he points out that this is grade-school level discussion. It's a continuation of CallItAsItIs's posts where he says things like "I cannot pack an entire radiometry textbook into this comment space". Long experience tells me that someone who pretends it is too complex or hard to explain things to me has reached a point where they are trying to hide their obvious lack of knowledge.
What is also glaringly obvious is that when CallItAsItIs reads pretty much anything, the only part that makes it into his mental model is any small snippet that he thinks confirms his misunderstandings. Anything else is rejected as "irrelevant".
More than once, I have referred to Schwarzschild’s equation, and linked to its discussion on Wikipedia. CallItAsItIs claims (in comment 791) "I have checked out every link and diagram that was posted, and only found two that were even remotely related to the problem I am addressing,"
So, what has CallItAsItIs's reaction to Schwarzschild’s equation? It's in comment 796 (quoted in its entirety, for context):
Amazing! CallItAsItIs has noticed that Schwarzschild’s equation includes both absorption and emission of radiation. But all he sees is the bit that he thinks confirms his "theory". For reference, here is equation, as posted on Wikipedia:
If we read further, we'll note that Schwarzschild’s equation is not applied to the atmosphere as a whole, but over small volumes where local thermodynamic equilibrium applies. Reading even further, we get to a section on "Application to Climate Science" that starts with (emphasis added):
In other words, you need to combine the local aspects of Schwarzschild’s equation into a series of equations that links many layers of the atmosphere - and also includes other forms of energy transfer besides radiation. Once you have Schwarzschild’s equation, there is still work to be done. The very next sentence on Wikipedia starts this:
Further down, we even get a section titled "Saturation". What do we find in the first paragraph? (Again, emphasis added).
Near the bottom of the Wikipedia article we see:
There is more there that disagrees with CallItAsItIs's "reading", but his Morton's Demon is blocking that information. He does not see that Schwarzschild’s equation includes emission of radiation that he deems "irrelevant" or non-existent. He does not see the information that should tell him that the local flux of IR radiation - including emissions - will be far more than just the IR radiation that has reached that altitude from the surface. He does not see that calculations of the effect of CO2 must look at more than just the strongest absorption band (and more than just radiation).
...but we have been trying to point all this out to CallItAsItIs for a week now.
It's very, very simple. In order for CallItASItIs's "interpretation" to be correct, one must ignore huge swaths of basic physics and observations of the climate system. And CallItAsItIs has been very effective at maintaining that ignorance in his knowledge. There is a word for that.
One more post for the moment. I alluded in my previous comment that to examining the effects of changing CO2 or other aspects of climate modelling, one needs to "combine the local aspects of Schwarzschild’s equation into a series of equations that links many layers of the atmosphere - and also includes other forms of energy transfer besides radiation".
One such study was the work of Manabe and Strickler (1964). Their figure 1 provides a useful illustration of what goes on inside such a model:
What we see is the results of four model runs, where atmospheric temperature changes over time. At the initial time, temperature is set to a somewhat arbitrary uniform temperature. Radiative transfer equations are used to evaluate the upward and downward fluxes (both IR and solar). At each altitude/layer in the model the energy balance is calculated, and the result is used to move to the next time step.
On the left, we see results if only radiative transfer occurs. There are two model simulations: one from a cold atmosphere, and one from a warm atmosphere. We see that it does not matter if the model started cold or warm - it converges on a common temperature profile.
The diagram on the left produces a tropospheric temperature profile that is too steep - a profile that would lead to extreme convection and cannot be sustained in a fluid atmosphere. On the right, we see the results when convection is added in, limiting the temperature profile. In essence, convection increases energy movement from the surface upward, so less needs to be transferred via radiation. More efficient energy transfer leads to the same total energy moving along a smaller slope (T vs. h) in the temperature profile.
What we also see on the right, is that such a model does a pretty good job of predicting global mean atmospheric temperature profiles. The model is verified by data.
A very similar model was used by Manabe and Wetherald 1967. Earlier in this discussion, I included their figure 16:
Notice that adding CO2 does not cause warming - oh oops, well, not at the top of the daigram. In the stratosphere, increasing CO2 leads to cooling. It's only when you get to the lower troposphere and surface that you see substantial warming.
So, when CallItAsItIs claims he can prove that CO2 can't cause warming, he does this by completely ignoring most of the physics.
[PS] Since Callitasitis continues to argue alternative physics, I propose all further commentary with him/her cease until equations are produced. Then predictions can be compared to reality. Since conventional physics makes accurate predictions for both earth and satellite measurements of radiation spectrum, as well as accurately predicting change in backradiation as CO2 increases, then the burden of proof is on CallitAsitis.
PS @805
Before making any rash decisions about ceasing further commentary with me, you might be interested in learning how the SkS claims about CO2 greenhouse warming violates the first law of thermodynamics. First, Kirchoff's Law is used to claim that for every photon absorbed by a CO2 molecule, a similar photon is emitted, and vice-versa. Now Kirchoff's Law applies only if the system is in thermal equilibrium, and a warming atmosphere is not in thermal equilibrium. Nevertheless, two SkS climate "experts" insist on applying it, and the result is that the number of 15 micron photons and the number of energized CO2 molecules never changes. This means that if there is any CO2 greenhouse warming, each such molecule would have to absorb a photon, deliver thermal energy to the surrounding N2 and O2 molecules, and still have enough energy to emit a similar photon. That, I'm afraid, is a violation of energy conservation!
Now, I know I have overstayed my welcome here, but you might want me around a little bit longer in case the "experts" have questions. Meanwhile, I will deliver my Fear No Carbon lectures if invited to do so, and they will include the issue I just raised.
[PS] As far as most physicists are concerned, the only problem is your understanding of how to apply physical laws. No further discusssion till you present an alternative that can be tested against observation. That is how science works. I reiterate - present your equivalent of heat flux through a slice of atmosphere.
https://github.com/atmtools/konrad and https://github.com/atmtools/arts have code you hack to remove what you consider is the flawed physics.
NO! -- You either re-post or respond to my satisfaction to my last comment (about violating the laws of thermodynamics) or we have nothing further to discuss.
[Snip]
Moderation complaints are always off-topic. Following the instructions of moderators is not optional.
Please note that posting comments here at SkS is a privilege, not a right. This privilege can be rescinded if the posting individual treats adherence to the Comments Policy as optional, rather than the mandatory condition of participating in this online forum.
Please take the time to review the policy and ensure future comments are in full compliance with it. Thanks for your understanding and compliance in this matter.
The argument that was being made by commenter CallItAsItIs (with which he apparently now feels confident enough to present within lectures!!) is to suggest that the likes of Kirchoff's Law can be ignored because the atmosphere is warming and thus Kirchoff's Law and its ilk which apply in a state of equilibrium do not apply under AGW. Of course, that situation should mean you adapt the physics such that they do apply, an adaption which commenter CallItAsItIs feels is not required as he can instead happily applies his own nonsense as an alternative.
The logical absence of an atmosphere in equilibrium under AGW has prompted me to set about calculating how large that out-of-equilibrium is under AGW and thus the significance of any deviation from equilibrium, this in a rough & wholly trivial manner. (I think I have managed to tame all the decimal points I've employed.)
A 15 micron photon has an energy of 1.3e-20J. Air at 1bar has a Cp of roughly 0.0012J/cm^3/K and is today warming at some 0.02 K/y or 6.3e-10 K/s, this with ECS=3ºC multiplying the warming by three. So this would suggest 2.5e-13W of forced warming, so requiring 20 million photons/second in the 15 micron band for a cc of air at sea level under today's AGW.
We can compare this roughly with the flux of such IR at average surface air temperatures, a flux of 400mW/m^2/cm^-1 in a band of width 170cm^-1 or 0.0068W/cm^2, roughly 500 quadrillion photons/second or 25 billion times the number of photons required for today's AGW.
The atmosphere is, of course, a little taller than this 1cc packet of air, with about 1.2 million times the mass of a sea level cc in the full cm^2 column, the full column requiring warming. About half the 500 quadrillion photons emitted at the surface are measured escaping from the TOA into space, the remaining 250 quadrilion maintaining the GH-effect. The AGW warming of the full column of atmosphere would require (pro rata with the sea level cc) 25 quadrillion photons, so about 10% additional to the GH-effect and 5% of the surface flux. That seems about right for a ballpark figure.
And while some may say that 10% is significant in terms of there being no equilibrium (and indeed AGW is significant), the statements of commenter CallItAsItIs are actually arguing inconsistently for/against the very existence of the 250 quadrillion photons/s/cm^2 required for a 33ºC GH-effect with an ECS=3ºC.
[PS] It is being left to CallitAsItis to define his/her physics and show that it conforms with observation. CallItAsItIs believe that their interpretation of physics is correct and we are obviously wrong. That fact that real physics correctly predicts observation and I cant see how CallItAsItIs can possibly explain observation with their physics should be reason to examine assumptions but no. However, we can look again when/if CallItAsItis has produced results. I see no further value in current arguments.
I apologize for my reaction in comment 807, but you must see my frustration with the general attitude with which I have greeted on this site. When are the people on this page going to realize that Beer's equation (ie., the differential equation we solve to obtain Beer's Law) is not my physics? It's been around much longer than I have. Neither is Schwartzschild's equation. Also, when are they going to realize that I am only studying the CO2 band saturation issue and not necessarily trying to win some best climate model award? Finally, when are they going to realize that when we focus a particular issue, we do our best to isolate that issue so that others don't affect observations and conclusions. Sure it's important to keep the "Big Picture" in mind, but one error in any of the critical smaller pieces could render the entire picture meaningless.
In regard to the Schwartzschild equation, I am already fully aware of everything stated in Bob Loblaw @804. In his comment, he quotes from the Wikipedia article titled Schwartzschild's equation for radiative transfer. In this article, the author describes a one-dimensional model in which the Schwartzschild equation is solved for the spectral density Iλ as a function of altitude s where the temperature T, CO2 molecular density n, and absorption factor \sigma are assumed to be given functions of s. This along with the value of Iλ at s=0 is all that is needed to solve the problem. Since the Schwartzschild equation is a first-order linear DE, its solution, generally involving integrals of known function which can be done numerically if necessary, has been "cut and dried" for well over a century. You can check this out for yourself, but I believe you will find negligible escape of energy for most any realistic input. Now, this approach may not be perfect, but isn't it more reliable than something that starts out by breaking the laws of thermodynamics?
You are still not getting the message. Until you show your math and provide full and complete numerical results, your comments will still be subject to strong moderation. None of your qualitative descriptions are correct.
You have been provided with link to code sources, and you have been pointed to Ramanathan and Coakley 1978 https://ramanathan.ucsd.edu/wp-content/uploads/sites/460/2017/10/pr15.pdf, where you can read about how this is done by climate scientists.
CallItAsItIs @809 :-
Wikipedia is far from perfect, but it is a usefully concise starting point in reviewing or broadening one's education. For instance, education regarding 3 important effects ~ (A) the Motivated Reasoning Effect ; (B) the Dunning-Kruger Effect ; (C) the GreenHouse Effect.
After giving deep consideration of these 3 matters, one is then justified in asking the question : Having proven to myself that I am right and all other scientists are wrong ~ why is it that the reputable scientific journals reject my scholarly paper demonstrating my findings?
Persecution cannot be the explanation ~ for the most eminent journals are actually keen to publish novel groundbreaking concepts (e.g. Relativity ; Quantum Mechanics ; DNA Structure ).
Each major journal welcomes iconoclastic breakthroughs : as does the Nobel Committee.
As previously stated, until such time as CallItAsItIs provides a proper mathematical description of how he thinks this should be done, along with numerical results, we would prefer that people not engage in further pointless discussion with his illusions.
Moderator @809-810
Yeh Sure! By the time I get those codes up and functioning on my computer, we will already know for certain if any of the global warming that you predict takes place. And even then I would have to figure out how to undo violations of energy conservation. No thank you!
But look at the bright side. You've made my Fear No Carbon lectures even more interesting when I show screenshots of this page.
[PS] Just show us your equivalent of the Ramanathan and Coakley equations for flux through a layer of atmosphere.
But if your theory cannot accord with observations (specificially backradation, spectrum, changes in spectrum and backradation as CO2 increases) and standard physics does, then your model of reality is wrong. Do the calculations correctly and there is no violation of conservation of energy but with your mental model of reality, you seem incapable of understanding that.
PS @811
Just show us your equivalent of the Ramanathan and Coakley equations for flux through a layer of atmosphere.
[Snip]
I did. It's the Schwartzschild equation shown along with its general solution in the Wikipedia article. Bob Loblaw also posted this equation at 804. You can get the backradiation from the non-exponential term in the solution, evaluating the indicated integral numerically if necessary. If you want actual number-crunching, I'll need a contract.
Wrong answer
PS @812
All right, let's do a little trouble-shooting to find my physics mistake. First, do we agree that temperature T, CO2 density n, and the spectral intensity Iλ (in the upward direction) all exist as smooth functions of 3D space between the surface and TOA? Next, do we agree that the Schwartzschild equation (posted in 804 and in Wikipedia) is a valid equation governing Iλ between the surface and TOA? We will start with those two questions and see what feedback I get.
PS @808
It is being left to CallitAsItis to define his/her physics and show that it conforms with observation.
Just what "observation" is "his/her physics" supposed to conform with? It wouldn't by any chance be observation predicted by "real physics". I'm sorry I had to bring this up, but I simply don't trust "experts" who can be shown that their physics violates energy conservation, and all they do is deny it. Please don't insult my intelligence any further!
[PS] The observation you need to match are the measurements of backradiation flux at surface, spectrum of that radiation, and the same as measured by satellites at TOA. Also the change in those measurements as CO2 increases. (eg https://www.nature.com/articles/nature14240) These match the theoretical calculation which you believe break conservation of energy. Let's see you do the same.