Is the CO2 effect saturated?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
The notion that the CO2 effect is 'saturated' is based on a misunderstanding of how the greenhouse effect works. |
|||||||
Climate Myth...
CO2 effect is saturated
"Each unit of CO2 you put into the atmosphere has less and less of a warming impact. Once the atmosphere reaches a saturation point, additional input of CO2 will not really have any major impact. It's like putting insulation in your attic. They give a recommended amount and after that you can stack the insulation up to the roof and it's going to have no impact." (Marc Morano, as quoted by Steve Eliot)
At-a-Glance
This myth relies on the use (or in fact misuse) of a particular word – 'saturated'. When someone comes in from a prolonged downpour, they may well exclaim that they are saturated. They cannot imagine being any wetter. That's casual usage, though.
In science, 'saturated' is a strictly-defined term. For example, in a saturated salt solution, no more salt will dissolve, period. But what's that got to do with heat transfer in Earth's atmosphere? Let's take a look.
Heat-trapping by CO2 in the atmosphere happens because it has the ability to absorb and pass on infra-red radiation – it is a 'greenhouse gas'. Infra-red is just one part of the electromagnetic spectrum, divided by physicists into a series of bands. From the low-frequency end of the spectrum upwards, the bands are as follows: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Gamma rays thus have a very high-frequency. They are the highest-energy form of radiation.
As our understanding of the electromagnetic spectrum developed, it was realised that the radiation consists of particles called 'photons', travelling in waves. The term was coined in 1926 by the celebrated physicist Gilbert Lewis (1875-1946). A photon's energy is related to its wavelength. The shorter the wavelength, the higher the energy, so that the very high-energy gamma-rays have the shortest wavelength of the lot.
Sunshine consists mostly of ultraviolet, visible light and infra-red photons. Objects warmed by the sun then re-emit energy photons at infra-red wavelengths. Like other greenhouse gases, CO2 has the ability to absorb infra-red photons. But CO2 is unlike a mop, which has to be wrung out regularly in order for it to continue working. CO2 molecules do not get filled up with infra-red photons. Not only do they emit their own infra-red photons, but also they are constantly colliding with neighbouring molecules in the air. The constant collisions are important. Every time they happen, energy is shared out between the colliding molecules.
Through those emissions and collisions, CO2 molecules constantly warm their surroundings. This goes on all the time and at all levels in the atmosphere. You cannot say, “CO2 is saturated because the surface-emitted IR is rapidly absorbed”, because you need to take into account the whole atmosphere and its constant, ongoing energy-exchange processes. That means taking into account all absorption, all re-emission, all collisions, all heating and cooling and all eventual loss to space, at all levels.
If the amount of radiation lost to space is equal to the amount coming in from the Sun, Earth is said to be in energy balance. But if the strength of the greenhouse effect is increased, the amount of energy escaping falls behind the amount that is incoming. Earth is then said to be in an energy imbalance and the climate heats up. Double the CO2 concentration and you get a few degrees of warming: double it again and you get a few more and on and on it goes. There is no room for complacency here. By the time just one doubling has occurred, the planet would already be unrecognisable. The insulation analogy in the myth is misleading because it over-simplifies what happens in the atmosphere.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
This myth relies on the use of a word – saturated. When we think of saturated in everyday use, the term 'soggy' comes to mind. This is a good example of a word that has one meaning in common parlance but another very specific one when thinking about atmospheric physics. Other such words come to mind too. Absorb and emit are two good examples relevant to this topic and we’ll discuss how they relate to atmospheric processes below.
First things first. The effect of CO2 in the atmosphere is due to its influence on the transport of 'electromagnetic radiation' (EMR). EMR is energy that is moving as x-rays, ultraviolet (UV) light, visible light, infrared (IR) radiation and so on (fig. 1). Radiation is unusual in the sense that it contains energy but it is also always moving, at the speed of light, so it is also a form of transport. Radiation is also unusual in that it has properties of particles but also travels with the properties of waves, so we talk about its wavelength.
The particles making up radiation are known as photons. Each photon contains a specific amount of energy, and that is related to its wavelength. High energy photons have short wavelengths, and low energy photons have longer wavelengths. In climate, we are interested in two main radiation categories - firstly the visible light plus UV and minor IR that together make up sunshine, and secondly the IR from the earth-atmosphere system.
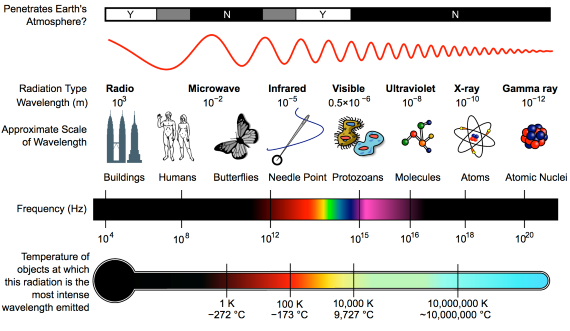
Fig. 1: diagram showing the full electromagnetic spectrum and its properties of the different bands. Image: CC BY-SA 3.0 from Wikimedia.
CO2 has the ability to absorb IR photons – it is a 'greenhouse gas'.So what does “absorb” mean, when talking about radiation? We are all familiar with using a sponge to mop up a water spill. The sponge will only absorb so much and will not absorb any more unless it's wrung out. In everyday language it may be described, without measurements, as 'saturated'. In this household example, 'absorb' basically means 'soak up' and 'saturated' simply means 'full to capacity'. Scientific terms are, in contrast, strictly defined.
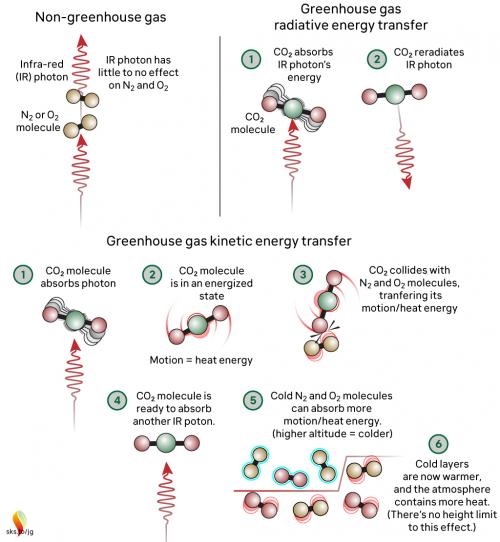
Now let's look at the atmosphere. The greenhouse effect works like this: energy arrives from the sun in the form of visible light and ultraviolet radiation. A proportion reaches and warms Earth's surface. Earth then emits the energy in the form of photons of IR radiation.
Greenhouse gases in the atmosphere, such as CO2 molecules, absorb some of this IR radiation, then re-emit it in all directions - including back to Earth's surface. The CO2 molecule does not fill up with IR photons, running out of space for any more. Instead, the CO2 molecule absorbs the energy from the IR photon and the photon ceases to be. The CO2 molecule now contains more energy, but that is transient since the molecule emits its own IR photons. Not only that: it's constantly colliding with other molecules such as N2 and O2 in the surrounding air. In those collisions, that excess energy is shared with them. This energy-sharing causes the nearby air to heat up (fig. 2).
Fig. 2: The greenhouse effect in action, showing the interactions between molecules. The interactions happen at all levels of the atmosphere and are constantly ongoing. Graphic: jg.
The capacity for CO2 to absorb photons is almost limitless. The CO2 molecule can also receive energy from collisions with other molecules, and it can lose energy by emitting IR radiation. When a photon is emitted, we’re not bringing a photon out of storage - we are bringing energy out of storage and turning it into a photon, travelling away at the speed of light. So CO2 is constantly absorbing IR radiation, constantly emitting IR radiation and constantly sharing energy with the surrounding air molecules. To understand the role of CO2, we need to consider all these forms of energy storage and transport.
So, where does 'saturation' get used in climate change contrarianism? The most common way they try to frame things is to claim that IR emitted from the surface, in the wavelengths where CO2 absorbs, is all absorbed fairly close to the surface. Therefore, the story continues, adding more CO2 can’t make any more difference. This is inaccurate through omission, because either innocently or deliberately, it ignores the rest of the picture, where energy is constantly being exchanged with other molecules by collisions and CO2 is constantly emitting IR radiation. This means that there is always IR radiation being emitted upwards by CO2 at all levels in the atmosphere. It might not have originated from the surface, but IR radiation is still present in the wavelengths that CO2 absorbs and emits. When emitted in the upper atmosphere, it can and will be lost to space.
When you include all the energy transfers related to the CO2 absorption of IR radiation – the transfer to other molecules, the emission, and both the upward and downward energy fluxes at all altitudes - then we find that adding CO2 to our current atmosphere acts to inhibit the transfer of radiative energy throughout that atmosphere and, ultimately, into space. This will lead to additional warming until the amount of energy being lost to space matches what is being received. This is precisely what is happening.
The myth reproduced at the top – incorrectly stating an analogy with roof insulation in that each unit has less of an effect - is misleading. Doubling CO2 from 280 ppm to 560 ppm will cause a few degrees of warming. Doubling again (560 to 1130 ppm) will cause a similar amount of additional warming, and so on. Many doublings later there may be a point where adding more CO2 has little effect, but recent work has cast serious doubt on that (He et al. 2023). But we are a long, long way from reaching that point and in any case we do not want to go anywhere near it! One doubling will be serious enough.
Finally, directly observing the specific, global radiative forcing caused by well-mixed greenhouse gases has - to date - proven elusive. This is because of irregular, uncalibrated or limited areal measurements. But very recently, results have been published regarding the deep reinterrogation of years of data (2003-2021) from the Atmospheric Infrared Sounder (AIRS) instrument on NASA's Aqua Satellite (Raghuraman et al. 2023). The work may well have finally cracked the long-standing issue of how to make finely detailed, consistent wavelength-specific measurements of outgoing long-wave radiation from Earth into space. As such, it has opened the way to direct monitoring of the radiative impact (i.e. forcing + feedback) of greenhouse gas concentration changes, thereby complimenting the Keeling Curve - the longstanding dataset of measured CO2 concentrations, down at the planet's surface.
Note: Several people in addition to John Mason were involved with updating this basic level rebuttal, namely Bob Loblaw, Ken Rice and John Garrett (jg).
Last updated on 31 December 2023 by John Mason. View Archives































 Arguments
Arguments





































Charlie_Brown @825
All right — This whole matter of Kirchoff's law seems trivially simple to me, so I guess I must be missing something. Therefore, I seek your great wisdom in order to understand this law and its implications correctly. Now, my understanding is that for each photon that is absorbed, an identical one is emitted, and vice-versa. So, if a CO2 molecule absorbes a photon and emits one exactly like it, how much of the energy from the absorbed photon is available as heat energy to warm the atmosphere?
I will leave this intact, even though it is not the numerical calculation we are asking for, but this is a prime example of how you continue to get things wrong.
Kirchoff's Law does not say what you imply here.
Charlie_Brown @825
Forget about conserving only radiant energy and remember that conservation of energy includes conduction and convection.
The problem with this claim is that it is only by EMR that energy enters and leaves the atmosphere. Conduction and convection only redistribute energy already within the atmosphere, and therefore give a sum total contribution of zero to the thermal energy contained in the atmosphere. I believe I explained this in one of my posts which the moderator removed.
[snip]
I think your blind spot is assuming that radiation in the CO2 absorption band is absorbed and disappears somewhere within about 10 meters of the surface.
No, that is not correct. I know that such thermal energy is somewhere within the atmosphere. It's simply a point that I haven't mentioned just yet since it is not relevant to solving the Schwartzschild equation. One thing worth pointing out, however, is that while adding massive amounts of CO2 to the atmosphere does not increase the overall CO2 temperature forcing, the entire radiated energy from the 15 micron band would be concentrated within just a few meters from the surface. From this, one might think that the surface would be too hot to touch! And that might be true except for one thing — convection. In addition to the surface being so hot, steep temperature gradients would form which would then result in steep pressure gradients. Then, according to the Navier-Stokes equation, the pressure gradient would drive a fluid velocity near the surface which carries away the excess heat. Anyway, I just wanted to make the point that I have not forgotten about that heat within 10 meters of the surface. It simply hasn't been a compelling issue just yet.
Leaving a portion of this intact. Once again, your assertion that no energy enters or leaves the atmosphere except by radiation is wrong. This is only true at the top of the atmosphere. It is not true at the surface. And this has been explained to you several times. Look at this diagram, once again.
Charlie_Brown @825
Forget about conserving only radiant energy and remember that conservation of energy includes conduction and convection.
The problem with this claim is that it is only by EMR that energy enters and leaves the atmosphere.
[snip]
Conduction and convection only redistribute energy already within the atmosphere, and therefore give a sum total contribution of zero to the thermal energy contained in the atmosphere. I believe I explained this in one of my posts which the moderator removed.
I think your blind spot is assuming that radiation in the CO2 absorption band is absorbed and disappears somewhere within about 10 meters of the surface.
No, that is not correct. I know that such thermal energy is somewhere within the atmosphere. It's simply a point that I haven't mentioned just yet since it is not relevant to solving the Schwartzschild equation. One thing worth pointing out, however, is that while adding massive amounts of CO2 to the atmosphere does not increase the overall CO2 temperature forcing, the entire radiated energy from the 15 micron band would be concentrated within just a few meters from the surface. From this, one might think that the surface would be too hot to touch! And that might be true except for one thing — convection. In addition to the surface being so hot, steep temperature gradients would form which would then result in steep pressure gradients. Then, according to the Navier-Stokes equation, the pressure gradient would drive a fluid velocity near the surface which carries away the excess heat. Anyway, I just wanted to make the point that I have not forgotten about that heat within 10 meters of the surface. It simply hasn't been a compelling issue just yet.
No matter how many times you say this, it is still wrong.
CallItAsItIs @827 ; @828 ; @829 ~
[snip]
You are outdoing yourself. Not two, but now three (3) repeated posts within 2 hours.
Still, it does supply some reassurance that you aren't an A.I.
No A.I. that I am aware of, would make such errors. Nor would an A.I. keep repeating the multiple scientific errors that you keep repeating.
An A.I. of the most modern sort, would change and adapt its responses, when those errors were pointed out.
This does not help.
I have been staying out of this, to let the moderators try to control the situation, but CallItAsItIs's latest diatribes continue to make the same basic mistakes that he started with, so I"ll attempt once more to point out his main errors.
The moderator reposted the Trenberth Energy diagram in response to comment 827. I had previously discussed this diagram in comment 772, over a week ago. CallItAsItIs is under the illusion that no energy enters the atmosphere except via radiation. In the diagram, we see 17 W/m2 entering the atmosphere from the surface via "thermals", and 80 W/m2 entering the atmosphere via "evapotranspiration". These values are not zero.
CallItAsItIs continues to misunderstand the importance of non-radiative energy transfers in the atmosphere, by saying they don't matter, as they just redistribute energy, not adding it. He's wrong about adding it, but he is also wrong about the importance of redistributing it.
Now, let's take another look at CallItAsItIs's misunderstanding of the Schwarzchild equation and Kirchoff's Law. Again, previously posted, we have Schwarzschild's equation:
This is a differential equation, telling us the change in radiation in a layer (infinitely thin, only ds units thick, as Calculus is wont to do).
There are two terms in it (in the middle form):
...but we have not really talked about what all the variables mean. Copying from the Wikipedia page:
n is the number density of absorbing/emitting molecules (units: molecules/volume)
σλ is their absorption cross-section at wavelength λ (units: area)
Bλ(T) is the Planck function for temperature T and wavelength λ (units: power/area/solid angle/wavelength - e.g. watts/cm2/sr/cm)
Iλ is the spectral intensity of the radiation entering the increment ds with the same units as Bλ(T)
Let's make a few points:
Although CallItAsItIs seems to accept that Schwarzschild's equation is reasonable, he rejects Kirchoff's Law, in spite of the fact that Schwarzschild's equation has Kirchoff's Law as one of its essential parts. In order to reject Kirchoff's Law he throws out bogus "laws of thermodynamics" and "thermal equilibrium" claims that have been criticized many times:
At this point, it is clear that CallItAsItIs suffers from two major intellectual issues:
I don't think there is much more we can do help him understand. The resistance is extremely strong.
Callitasitis at 826:
This is the basis of your misconceptions. When a CO2 molecule absorbs a photon it does not emit another photon. The energy from the photon is distributed to other molecules. New photons are emitted according to the temperature where they are emitted. You are focused on the incorrect issue and you do not understand how energy flows through the atmosphere. IndividuaI photons are not important. I will try to explain it to you again.
The surface of Earth emits radiation according to its temperature. Let us say it is 290K. I will look at the later of air 1000 meters high. As you point out, all the upwelling energy is absorbed. Since I am now higher this layer is colder, it is only 285K. I look at this layer from above. I see that this layer emits 15 micron radiation according to its temperature. Since it is colder than the surface it emits less energy.
Now I look at the next 1000 meters of the atmosphere. It absorbs all the upwelling radiation. It is only 280K so it emits less upwelling radiation.
This process goes up to the top of the atmosphere with each layer emitting less energy up. The incoming energy from the Sun makes the Earth about 275K. At the top of the atmosphere the energy emitted averaged over all wavelengths of radiation must be 275K due to the law of conservation of energy. Incoming energy must equal outgoing energy. For the purpose of this discussion I will say that at 3000 meters the temperature is 275K.
The key issue is the concentration of CO2. The top of the atmosphere is determined by the concentration of CO2. The concentration of CO2 determines the amount of energy emitted up. If the concentration of CO2 is lower the TOA is lower, if the concentration of CO2 is higher the TOA is higher. The concentration of CO2 is lower when you go up in the atmosphere because the pressure is lower.
Now I add more CO2 to the atmosphere. I do not care about the first 2999 meters of the atmosphere, I only care about the top of the atmosphere
At 3000 meters there is now more CO2 so more upwelling energy is absorbed. (upwelling energy from 2,999 meters). This reduces the energy emitted to space. In order to conserve energy the top of the atmosphere has to move higher where CO2 is lower. Now it is 275K at 3,100 meters instead of 3,000 meters. The top of the atmosphere must stay 275K to conserve energy. The CO2 concentration is lower since the pressure is lower.
The temperature of the atmosphere is determined by the lapse rate. The lapse rate is a physical property of the atmosphere. It is measured as 5K per 1000 meters. That means that if you go up 1000 meters the air is 5K colder.
When it was 275K at 3,000 meters it was 290K at the surface. Now, because the CO2 has increased, it is 275K at 3,100 meters. Since the top of the atmosphere is 100 meters higher the lapse rate forces the entire atmosphere to increase by 0.5K. It is now 290.5K at the surface.
The top of the atmosphere is extremely sensitive to the concentration of CO2. It doesn't matter that the first 10 meters on the atmosphere absorb all upwelling 15 micron radiation from the surface. The upwelling radiation is replaced by black body radiation originating in the atmosphere. Focus on the top of the atmosphere.
You have not taken advanced atmospheric physiior chemistry. Reading a little on the Internet does not make you smarter than all the scientists in the world. Accept that you do not understand the energy flow in the atmosphere.
Response @825
... would bother to try to learn it.
As a Ph.D. physicist, I have already learned the fundamental principles claimed in this AGW stuff, and I resent this comment of yours. The issue at hand is that after careful review of the SkS "rebuttals" to the CO2 band saturation effect, I believe they violate at least one very fundamental physical principle and am trying to get this matter resolved. And your snip-happy attitude isn't helping any.
CallItAsItIs:
You need to ask for your money back.
CallItAsItIs @832
If you understand the fundamental principles, then perhaps you are not understanding how the parts of AGW fit together and do not violate any of them. Help us out here. Just what part of AGW is it that you think violates a fundamental principle? Kirchhoff’s Law, which I thought was your stumbling block, was well covered in great detail by Bob Loblaw @830 and Michael Sweet @831. Perhaps you are confused about the word “atmosphere” as defining the system energy balance. @827 you say:
“The problem with this claim is that it is only by EMR that energy enters and leaves the atmosphere. Conduction and convection only redistribute energy already within the atmosphere, and therefore give a sum total contribution of zero to the thermal energy contained in the atmosphere.”
It is correct that only radiant energy enters and leaves the overall global system at the Top of the Atmosphere (TOA) where the global system includes the atmosphere plus the surface. However, you seem to exclude the surface by saying that conduction and convection only redistribute energy already in within the atmosphere. As shown in the energy budget diagram, convection and evaporation transfer energy from the surface to the atmosphere. Total IR flux leaving the TOA is determined by the temperature profile of the atmosphere and the surface. The intensity of the total IR spectrum at the TOA is integrated to give IR heat flux. Do not think that AGW is caused only by absorbing IR in the lower atmosphere. It is caused by reducing IR loss to space which then has to be compensated by accumulating energy until IR from a warmer surface balances the reduction through the full CO2 absorption band. The warmer surface heats the lower atmosphere.
Response @827
Well, you will be pleased to know that every energy flow shown in your diagram is EMR — just different frequencies. And since our topic is the CO2 greenhouse effect, we are only interested in the absorption band from 14-16 microns. Finally, since we are not in the realm of nonlinear optics, the law of conservation of energy applies to each and every frequency individually as well as collectively. I hope clears up your understanding and perspective on this issue.
I'd be tempted to ask what frequency evaporation happens at, but I know there isn't an answer to that, because it isn't EMR.
The only understanding you have cleared up is that you are eternally misinformed.
CallItAsItIs @ 835
Unfortunately, I am completely lost by this explanation. We are talking about the Energy Budget diagram @753 and @827, aren’t we? Thermal is convection from the surface, 17 W/m^2. Evapotranspiration is 80 W/m^2, and Latent heat for condensation is 80 W/m^2. Those energy streams redistribute energy in the lower atmosphere and affect the atmospheric temperature profile, along with the lapse rate. The Response @827 already explained it correctly. Note that an energy budget describes the energy flows within the overall global system. If you are talking about the overall global system energy balance, then the boundary is at the TOA. The intermediate streams in the lower atmosphere are not needed. The energy balance becomes:
Solar In (341 W/m^2) = Solar Reflected (102 W/m^2) + IR Out (239 W/m^2)
IR Out is the full IR spectrum because it includes IR emitted by the surface that is not absorbed and re-emitted by greenhouse gases.
Conservation of energy does not apply to each and every frequency individually. Kirchhoff’s Law explained @756 and elsewhere allows for collisions between molecules and energy exchange by conduction. For a small, localized packet of isothermal atmosphere, absorptance will equal emittance. But since there is a temperature change with altitude, it is conservation of energy, not conservation of photons.
It is clear that you did not understand my description of AWG because you say that we are only interested in 14-16 microns. But AWG also includes strengthening of weak CO2 emittance lines between 13-14 microns and 16-17 microns, as illustrated @788. And warming of the surface increases caused by increasing CO2 increases IR from all of the transparent lines also. The atmospheric spectrum was shown in @819 and for different altitudes @731.
My perspective now is that you have no business critiquing AGW because we are not even close to talking about the same thing. We seem to be hopelessly talking past each other.
Charlie Brown:
Your points to Callitasitis are on point
I think your addition has an error. From the diagram at 827:
341.3 (solar in) =
101.9 (reflected) + 238.5 (ir out) +0.9 (absorbed)
The absorbed is the energy that heats the Earth. When you rounded the numbers they didn't add up. I do that all the time.
Callitasitis:
Your claim at 835 that frequency is conserved directly contradicts your previous claim that all the 15 micron photons are absorbed in the lower atmosphere. Making contradictory claims voids your entire argument
In any case the claim frequency is conserved is obviously false. Incoming energy is visitlight while outgoing energy is IR light. Frequency is obviously not conserved.
I cannot believe that someone with a physics degree would make such an obvious, basic error.
Michael Sweet @837
Thanks. I wondered about that imbalance in the budget. I didn’t know if it was the difference between input and outputs or whether it was calculated from the radiative balance. Radiant energy calcs are precise, so I prefer using MILIA for that purpose or using the forcings from the ICPP. A small difference between large numbers with uncertainties is not so precise. Anyway, it is the right order of magnitude for the calculated radiative imbalance.
No more please. Attempting to explain physics to Callitasitis has been futile. He/she needs to show how their wierd way of thinking reproduces existing measurements or better still correctly predicts measurements of radiation. So far Callitasitis has resisted tying their calculations to measurements and this thread is pointless till that happens.
michael sweet @831
When a CO2 molecule absorbs a photon it does not emit another photon. The energy from the photon is distributed to other molecules.
Agreed!, but Bob Loblaw, Charlie_Brown, and a few others seem to feel I am breaking Kirchhoff's Law in making such a claim. Could you maybe straighten them out in this matter.
This has been explained to you many times in this thread.
CallItAsItIs @840,
Perhaps we can work from that apparent point of agreement.
To correct the quote @831 a little
The reason for this is because the average relaxation time required for the excited CO2 molecule to emit another photon is many times longer than the average time before it will be in collision with other air molecules which will neutralise the excitation and thus convert the excitation energy into thermal energy within the gas mollecules.
So CallItAsItIs, I would assume someone with a PhD in Physics will have no problem with wrapping their head round the amended quote and the explanation and give it a yea or ney (and if 'ney' the reason why).
(And apologies to Moderators for this late attempt to reach some agreement.)
MA Rodgers @808
... and thus Kirchoff's Law and its ilk which apply in a state of equilibrium do not apply under AGW. Of course, that situation should mean you adapt the physics such that they do apply, ...
Now who's changing laws of physics!?
Everybody except you knows the answer to that question.
michael sweet @838
Your claim at 835 that frequency is conserved directly contradicts your previous claim that all the 15 micron photons are absorbed in the lower atmosphere. Making contradictory claims voids your entire argument.
What do you mean "frequency is conserved"? I never made any such claim, not in 835 nor any place else!
You don't even know what it means when you say "the law of conservation of energy applies to each and every frequency individually", do you?
MA Rodger @841
Your correction to the quote at 831 certainly seems reasonable.
CallItAsItIs @842,
That was not a response to my question @841 which, posted eight minutes before your comment @842, appears to have prompted your comment @842 as a reply.
You ask "Now who's changing laws of physics!?" Do note the quote you read @808 present @842 was my interpretation of your argued position. The full quote runs:-
If you feel AGW is adding some fundamental problem for the use of Kirchoff's Law, perhaps AGW can be ignored for this description of the physics of the atmosphere. Consider instead the pre-industrial pre-AGW climate: does that fix your objection to the use of Kirchoff's Law?
And note the more trivial question I posed @841remains unanswered.
Until such time as CallItAsItIS provides a numerical calculation of the purported effects he claims exist, and shows that it agrees with measurements, expect any and all comments from CallItAsItIs or reacting to him to be deleted.
[snip]
Well, I believe I have found the source of much of our contention lately. It turns out that there are two versions of Kirchhoff's Law out there — the one Kirchhoff actually wrote and the that's in general use by climate scientists. We will call that one the KirchhoffCS Law. I won't get into details on the actual Kirchhoff Law, but it basically states that for an emissive blackbody (or "graybody") emissivity = absorptivity (ie. how well the body emits is also how well it absorbs). There is a well-written article on it in the WikiPedia. The KirchhoffCS Law states that for such a graybody, there must be a photon emitted for each one that is absorbed and vice-versa. This "law" however, has a special property which I've shown earlier — It violates energy conservation! So is it valid or invalid? You tell me!
Now this is where it starts to get interesting. From comment 729 onward, Charlie_Brown and Bob Loblaw claim that I failed to apply "Kirchhoff's Law" and therefore my results are incorrect. Kirchhoff's Law, however, is not used in the calculation of the absorbed IR radiation, so I don't know what they mean by "apply". My particular model consists simply of the Schwartzschild equation with given values of the solution at the surface along with given temperature and absorption profiles from the surface to the TOA. This turns out to be a fully defined and well-posed problem that is consistent with Kirchhoff's Law (ie. the real Kirchhoff's Law) without having to somehow "apply" it. It's analogous to solving a classical mechanics problem with Newton's Laws. In this case, it is only Newton's Laws that are used in the actual calculations. Assuming they are done correctly, the results would be consistent with energy conservation (except possibly for losses due to friction) without any additional steps to enforce energy conservation.
Anyway, I got my wake-up call about this Kirchhoff's Law duality from Bob Loblaw @830 when he states that
Kirchoff's Law does not say that absorption = emission. It just says that the efficiency of absorption is equal to the efficiency of emission.
which is an obvious denial of the KirchhoffCS Law even though he and Charlie_Brown had already used it a bunch of times (@729, @735, @737, @756, @786, @799) and continue to do so in their claims that my results showing saturation of 15 micron absorption band were erroneous. So the question now is
Why is it that this "law" has been shoved in my face since comment 729 knowing full-well it is false!?
Unfortunately, I think I know the answer to this question. The extra photons claimed by KirchhoffCS are essential for continuing the upward-bound 15 micron radiation above what would otherwise be the extinction altitude. Then, since we are not yet at the TOA, there is still plenty of CO2 for more absorption and warming. This is the basis for the claim made in the SkS Basic Rebuttal that
... there is always IR radiation being emitted upwards by CO2 at all levels in the atmosphere.
Now, between this and the repeated personal attacks and stonewalling of my arguments with irrelevant issues, I no longer view this AGW song-and-dance as simply bad science. It's intentional deception! And I will regard it as such in any lectures I may give and in communications with my Congressmen.
Still waiting for your equations and numerical results.
[PS] I will settle for your versions of the Ramanthan and Coakley equations for radiative flux through a layer. Should be too hard for a PhD in physics -remove the bits you dont like. Bet they dont predict measurements. Till you match your fantasies to real world observation, noone is interested in your problems with comprehension.
[snip]
Response @835
Evaporation occurs from the water surfaces which are not part of the atmosphere and therefore contributes zero energy. What does happen in the atmosphere is the release of 80 W/m3 of latent heat at precipitation, and this heat energy is EMR which is then absorbed by the air molecules, thereby raising temperature. In my model, the effect of this heat would be taken into account by adjusting the temperature profile accordingly.
Also, I am glad you were only tempted to ask, because if you had, then I would be asking for an apology for your next comment.
Still not providing the requested equations and numerical results.
[snip]
Response @826
Kirchoff's Law does not say what you imply here.
The climate science version of Kirchhoff's Law says exactly what I imply here. It turns out that there are two versions of this law out there, the one written by Kirchhoff and the one used by climate scientists, and it is the latter version that is needed to "debunk" the CO2 band saturation effect. This was already explained in a previous posting of mine, but you removed it!
Still not providing the requested equations and numerical results.
CallItAsItIs,
I note your comment @844 replying to my comment @841, crossed with my own @845, so the ball is perhaps in my court.
And perhaps I should be sure to give you the chance of providing a numerical answer in this to-&-fro.
As you do not appear to be a numbers sort-of-guy (which is strange for a physics PhD), I would be surprised if you could put some sort of value on the proportion of υ2-excited CO2 molecules in air that would emit a photon in the 15 micron band as the "numerical calculation" to demonstrate such a proportion is majorly complex & difficult. I've seen a proportion of 1-in-50,000 simplistically calculated. But the take-away is that it is massively small as, simplistically, the average relaxation time for an excited CO2 molecule to emit a 15-micron photon is measured in tenths of a seconds while such a CO2 molecule will be on average impacted by other air molecules in a matter of microseconds. Thus this transmission of excitation via photon→CO2→photon cannot by itself satisfy Kirchoff's law αλ = ελ.
And it also does not explain the measure 15-micron IR flying round the atmosphere.
But let us not get hung-up on the applicability or otherwise of Kirchoff's law.
You seem entirely positive about the applicability of Schwartzschild's equation, telling us @823 "the Schwartzschild equation is the correct equation to solve in order to determine the spectral intensity Iλ." Schwartzschild's equation (dIλ/ds = nσλ[Bλ(t)-Iλ]) tells us Iλ will not be effectively snuffed out when the path-length of the IR from the ground is reached. Rather, with constant temperature Iλ is also constant (Iλ = Bλ(t) = Planck's Constant) and in the atmosphere Iλ will slowly drop with the lapse rate (we can calculate it as just a 0.13% drop in 10m which is longer than the path length of 15-micron radiation at sea level, and a 50% drop by about 5,000m), and on until CO2 thins allowing the path-length to reach infinity with IR being shot out into space. (Note that Kirchoff's law differs from this situation solely because it applies to a constant temperature.) So to satisfy both the Schwartzschild equation (and Kirchoff's law), we need a new source of excited CO2 molecules to prevent the IR flux being snuffed out so quickly.
So, to set you a question CallItAsItIs with a numerical answer.
Given both Schwartzschild's equation and measurements show there is no extinction of 15-micron IR in the atmosphere, there must be some extra source of υ2-excited CO2 molecules. Therefore, how big is that extra source of excited CO2 relative to the IR as a source? Roughly?
(As a PhD-wielding engineer, this extra source ???→CO2→photon comes as no surprise to me.)
MA Rodger @849
I re-examined the arguments behind Kirchhoff's Law, and determined that it is not applicable to my study of greenhouse warming for the simple reason (which I have stated before but no one seems to take seriously) that a warming system is not in thermal equilibrium. In the case of greenhouse warming, each atmospheric slice contains GHG molecules which absorb IR energy originating from outside the slice, and therefore they are not isolated. Hence, Kirchhoff's Law does not apply in my study, neither the correct version nor what I have called the "climate science" version, and therefore claims of my "ignoring Kirchhoff's Law" are invalid.
Also, in regard to me being or not being a "numbers sort-of-guy", I certainly was at one time, but have not kept up very well with new developments and software tools since my career fizzled. I believe I still have excellent mathematical and analytical skills, but I do tend to "cut corners" or look to others when it comes to actual number crunching.
Note to moderator:
Please do not remove this comment at least until MA Rodger has had a chance to review it.
[PS] MA Rogers comment was left so you could respond.
Now give us the equations that relate to what can be meaured. MA Rodgers. same stuff. Nothing more please till we something testable.