How we know the greenhouse effect isn't saturated
Posted on 13 February 2014 by Glenn Tamblyn, jg
This is the Basic rebuttal to the myth 'CO2 effect is saturated'
The mistaken idea that the Greenhouse Effect is 'saturated', that adding more CO2 will have virtually no effect, is based on a simple misunderstanding of how the Greenhouse Effect works.
The myth goes something like this:
- CO2 absorbs nearly all the Infrared (heat) radiation leaving the Earth's surface that it can absorb. True!
- Therefore adding more CO2 won't absorb much more IR radiation at the surface. True!
- Therefore adding more CO2 can't cause more warming. FALSE!!!
Here's why; it ignores the very simplest arithmetic.
If the air is only absorbing heat from the surface then the air should just keep getting hotter and hotter. By now the Earth should be a cinder from all that absorbed heat. But not too surprisingly, it isn't! What are we missing?
The air doesn't just absorb heat, it also loses it as well! The atmosphere isn't just absorbing IR Radiation (heat) from the surface. It is also radiating IR Radiation (heat) to Space. If these two heat flows are in balance, the atmosphere doesn't warm or cool - it stays the same.
Lets think about a simple analogy:
What might we do to increase the water level in the tank?
We might increase the speed of the pump that is adding water to the tank. That would raise the water level. But if the pump is already running at nearly its top speed, I can't add water any faster. That would fit the 'It's Saturated' claim: the pump can't run much faster just as the atmosphere can't absorb the Sun's heat any faster
But what if we restricted the outlet, so that it was harder for water to get out of the tank? The same amount of water is flowing in but less is flowing out. So the water level in the tank will rise. We can change the water level in our tank without changing how much water is flowing in, by changing how much water is flowing out.
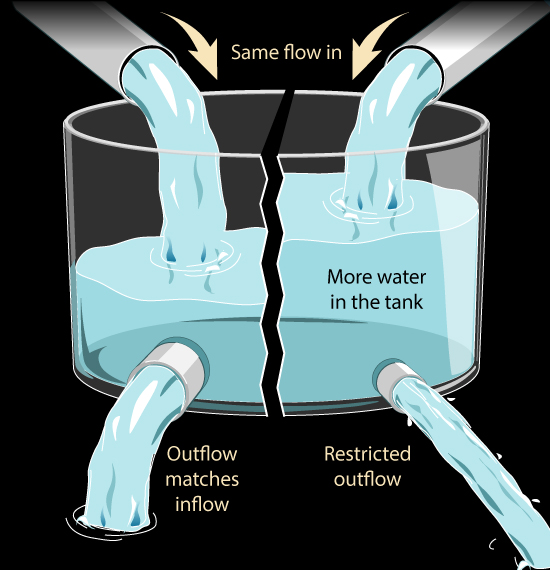
Similarly we can change how much heat there is in the atmosphere by restricting how much heat leaves the atmosphere rather than by increasing how much is being absorbed by the atmosphere.
This is how the Greenhouse Effect works. The Greenhouse gases such as carbon dioxide and water vapour absorb most of the heat radiation leaving the Earth's surface. Then their concentration determines how much heat escapes from the top of the atmosphere to space. It is the change in what happens at the top of the atmosphere that matters, not what happens down here near the surface.
So how does changing the concentration of a Greenhouse gas change how much heat escapes from the upper atmosphere? As we climb higher in the atmosphere the air gets thinner. There is less of all gases, including the greenhouse gases. Eventually the air becomes thin enough that any heat radiated by the air can escape all the way to Space. How much heat escapes to space from this altitude then depends on how cold the air is at that height. The colder the air, the less heat it radiates.
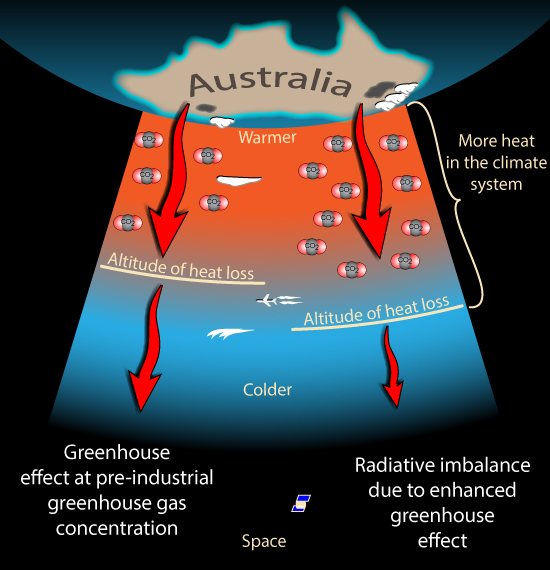
(OK, I'm Australian so this image appeals to me)
So if we add more greenhouse gases the air needs to be thinner before heat radiation is able to escape to space. So this can only happen higher in the atmosphere. Where it is colder. So the amount of heat escaping is reduced.
By adding greenhouse gases, we force the radiation to space to come from higher, colder air, reducing the flow of radiation to space. And there is still a lot of scope for more greenhouse gases to push 'the action' higher and higher, into colder and colder air, restricting the rate of radiation to space even further.
The Greenhouse Effect isn't even remotely Saturated. Myth Busted!































 Arguments
Arguments






























DM@50
I don't have a clue what you mean or think you mean when you use the term "attenuation". Maybe you could use a different word or words?
DM: "It doesn't really matter how much IR is attenuated in the "opaque layer" as some of the energy of the absorbed photons will still be transmitted to the "translucent layer" via collisions, convection and re-radiation."
mgardner: What exactly do you think "attenuation" means, other than that?
mgardner, I think Dikran is using "attenuation" to refer to absorbtion of the photon. When an IR photon (of the correct frequency) interacts with a CO2 molecule, the photons energy is transferred into a vibration of the atoms within the molecule, in the case of Global Warming this is primarily a bending motion. The photon (which is essentially a packet of energy) is destroyed, and the CO2 molecule starts vibrating more energetically. Thus the photon has been "attenuated".
At some later stage the CO2 molecule will relax into its "slower" vibration; either by emitting a new photon (back or outbound radiation), or by collisions with other molecules.
This response glosses over the quantum mechanics somewhat.
mgardner, a bit if politeness wouldn't go amiss, KR and I are only trying to be helpful.
Consider a photon emitted by the surface. It is absorbed by a molecule of CO2 in the "opaque layer", however the energy in that photon is transferred by molecular collisions to the translucent layer, where it is emitted upwards by another molecule of CO2 and it leaves the Earth. Now you could say that the opaque layer has attenuated the flow of IR photons, by absorbing this one, but has it attenuated the energy radiated into space? No, becase the energy was transformed, passed on to the translucent layer and then radiated into space. The fate of IR photons leaving the surface of the Earth is essentially irrelevant to the Earths energy budget, as virtually all of them (in the relevant absorption bands) are absorbed by the atmosphere at some point.
Asking about the absorption of photons by the opaque layer suggests you have an incorrect model of the nature of the greenhouse effect. It is what happens at the top of the atmosphere that determines the greenhouse effect, not by what happens near the surface.
As it happens, I am an electronic engineer by training, and I know exactly what attenuation means, the question is attenuation of what, the individual photons (which are irrelevant), or the energy that they carry (which isn't)?
DM (and Phil)
Yes, that's exactly the question, and it should have been obvious, if you had read my language carefully, that I clearly distinguish between "absorption of all possible photons from the emitting plane" (opacity, which is the essence of the original myth about 'saturation',) and "a reduction of the energy emitted from that plane as it traverses the interval" (attenuation). I don't use words randomly.
Which brings us to the question of what a polite response to someone asking a serious question might be. My approach is to make sure I understand the question by asking for clarification rather than lecturing and repeating the same thing multiple times. Then, if I couldn't provide the answer, I would say "I don't know" rather than deflecting by saying "you're missing the point".
So, I'll repeat my questions:
If I understand correctly, we take something like 5km to be the altitude above which attenuation of IR by CO2 is negligible--"where energy radiates freely to space".
Now, without telling me about how it all varies with season and latitude, and all of the complications involved in doing the calculations, can I find out about what that altitude would be if we double the CO2 concentration? Is it 100m higher, or 1km higher, or 4 km or what?
I would also like to know, again, order of magnitude, what the thickness of an 'opaque' 'layer' just below that original 5 km altitude would be. And what the attenuation would be for IR radiation in some CO2 band through that 'layer'.
There's no shame in admitting "I don't know"; that's what I'm doing by asking these questions. But it should be obvious if you are paying attention that I know what I'm asking about.
It would raise the emission height a few hundred meters.
scaddenp that is a very interesting diagram. It was already my understanding that there wasn't really an "opaque layer" and a "translucent layer" with a distinct boundary, but I had no idea that the distinction was anything like as diffuse as is suggested by that diagram. In that case, is probably better not to think of it in those terms, other than as a very crude qualitative model.
mgardner @54:
Being a layman like you this discussion is quite challenging and enlightening also for me.
Regarding your three underlined paragraphs / questions I’ll try to provide some information.
1. There isn’t a specific altitude there IR attenuation by CO2 or any other greenhouse gas becomes negligible. This depends strongly on the IR wavelength, and varies from sea level in the “atmospheric window” (8-12 microns) to the lower or middle of the stratosphere (25-30 km) in the part of the IR spectrum most strongly absorbed by CO2 (close to 15 microns). The 5 km figure is just an average of this. If CO2 was the only greenhouse gas, the average altitude of heat loss would be much lower (1-1.5 km?) because its absorption is negligible outside the wavelength range 13-17 microns. IR radiation outside this range would therefore escape to space directly from the surface.
2. The forcing from a doubling of CO2 is usually given as about 4 watts/m2, meaning that it would reduce the outgoing longwave radiation (OLR) from 240 watts/m2 to 236 watts/m2 until surface warming restored the balance by increasing the OLR to its previous level.
240 watts/m2 corresponds to 255 K, so how much cooling is needed to reduce the radiation to 236 watts/m2?
According to the Stefan-Boltzmann law, 236 watts/m2 corresponds to 254 K, so a 1 K cooling is sufficient to reduce the OLR by 4 watts/m2. With a lapse rate of 6.5 K per km the answer to your question should be about 150-160 m.
Surprising? A doubling of CO2 raises the average altitude of heat loss by merely 150-160 metres?
Don’t forget that most of the IR spectrum isn’t affected at all, while the heat loss from the central part of the CO2 absorption band will rise several kilometres. And the water vapour feedback isn't included here.
3. Sorry, it’s past midnight here in Norway now. I’ll try to write something about the thickness of opaque layers tomorrow if nobody else has anything about that.
scaddenp - I would agree with Dikran; that's a very useful diagram, and that the spread of emission altitudes is rahter larger than I thought.
One of the more frustrating points of this exchange (and I think where some of the confusion and talking past one another has arisen) is the repeated reference to 'opaque' and 'translucent' layers - as I tried to point out above there is varying IR emission from a range of altitudes, not a single transition altitude between two distinct states. IR in GHG bands is emitted to space at a range of different altitudes - with the sum of that emission adding up to the oft-noted temperature equivalent profiles seen in satellite observations of those bands.
Gentlepeople:
Clearly, a simple explanation for radiation transfer is not easy. I'll try to explain some basics and help show where Dikran and scaddenp are coming from.
First of all, photons that have been emitted will follow Beer's Law, which states that absorption will follow this differential equation:
dI = -b dz
where I is radiation intensity, z is distance (we'll think height here for now), and b is the absorption coefficient. In the usual calculus form, dI is thus the change in intensity, dz is the change in distance, and the minus sign tells us that the intensity is decreasing as radiation is absorbed.
If b is a constant, it is easy to integrate this equation over a distance, and we get:
I/I_0 = exp(-bz)
where now I is the current I, I_0 is the I we started with at z=0, and z is now the final value of z. This integrated form of Beer's Law is the one most people will be familiar with. If we are thinking in terms of surface-emitted radiation reaching the top of the atmosphere, I_0 is the surface emission, and I is the amount reaching the top of the atmosphere (at height z) directly.
The "saturation" argument looks at I/I_0 and says it's very small for the value of b at normal CO2, and argues that making b larger won't really make I/I_0 significantly smaller. This is the "truthiness" of the argument, but it fails to look at what happens at other altitudes/intervals.
Let's take a crude example, where we go from 0 to 70 for distance, and use b=0.1 and b=0.2. (I pull these numbers out of thin air.)
We get, for I/I_0:
z I/I_0 (b=0.1) I/I_0 (b=0.2)
0 1 1
10 0.368 0.135
20 0.050 0.002
50 0.007 0.00045
70 0.0009 0.00000008
At z=70, the "saturation" argument says "who cares about the difference between 0.0009 and 0.00000008? [b = 0.1 vs. b=0.2]"
At z=10, the atmosphere says "I care".
The point is that - even though little surface-emitted IR actually reaches the top of the atmosphere directly, increasing CO2 will have an effect on how much radiation is being absorbed in different parts of the atmosphere. Increasing the absorption rate (b) means that more IR is absorbed at lower heights, and you have to go through more absorption/reemission cycles (gradually moving upwards)before IR is radiated to space. At each reemission, only half is directed upwards (half goes downwards), so the number of absorption/reemission cycles affgects the efficiency with which the atmosphere get energy from the surface to space (more cycles - less efficient).
Now, if that isn't confusing enough, the total amount of IR radiation going upwards at height z is not solely based on how much surface radiation reaches that height. It also depends on:
- how much IR radiation is being emitted at height z in an upward direction. (This depends on temperature, which is not solely a radiation transfer result.)
- how much radiation has reached height z from all the other infinitely-thin layers of the atmosphere below height z, and has not been absorbed by all the layers in between.
The result, at height z, is a probability density function of where in the atmosphere (below z) that the IR radiation originated. Repeat for all values of z from 0 to the top of the atmosphere. Continuing from height z to the top of the atmosphere tells you how likely a given photon will escape directly to space from that height.
Now if that isn't confusing enough, the value of b is not a single value - it depends strongly on wavelength. All the equations must be applied at each individual wavelength, not for all wavelengths in total. After you've done each individual wavelength, you can then sum to get totals for a range of wavelengths (scaddenp's graph at 6:55AM).
This is what programs like MODTRAN will do for you. It will also not assume that b is constant with height, and other marvellous refinements (such as probably not using linear distance in Beer's Law).
And the end result is that I/I_0 for z=top of atmosphere (the "saturation" argument value) just doesn't tell use what we need to know about IR radiation transfer in the atmosphere, and that increasing CO2 will have an effect.
Another item that has been asked about: the relative role of convection vs. radiation in the atmosphere. In a (much) earlier comment on another thread, I provided a reference to an old paper by Manabe and Strickler (1964). Figure 1 of that paper is:
The left hand side shows a (modelled) atmosphere with no convection. The right hand side shows the same model with convective adjustment (which basically forces the model lapse rate to the observed tropospheric lapse rate). As you can see, convection in the troposphere is very significant. In the stratosphere, radiation dominates.
(The figure shows how the model converges over time to an equilibrium state as energy fluxes balance out.)
[BL] External image no longer available replaced with a copy at SkS [2022-08-15]
scaddenp@55
Thank you for directly answering my question. I zoomed in on the graph and used an index card on the screen to get ~900 m for the height difference between 280ppm and 400 ppm at .4 W/m2. (Is that about right?) That would make a temp difference around 6 K?.
Holding everything constant.
I'm going to reply to others on this as soon as I get the image insertion thing to work. That will require a bit more coffee.
Bob Loblaw et al:
First, I'd like to explain why I get testy about some responses.
I'm a Spherical-Cow-Physics kind of guy. In learning, problem solving, and in teaching, I do (did) as I was taught a long time ago and try to build understanding from the ground up. Now, I realize that's my particular style, and there's no obligation for anyone to respect it. However, it is a matter of principle when it comes to teaching practice-- 'setting up the problem' requires figuring out the 'position' of the student before 'applying forces to achieve desired displacement'.
In this case, I'm playing the role of the student, but I'm getting this adversarial and dismissive attitude about my questions. Perhaps it's understandable since you've been dealing with 'skeptics' a lot, but even when I think someone is likely a 'skeptic', I ask questions to pin down where he's coming from, rather than just firing off the standard litany and telling him 'you just don't get it' and 'your questions are irrelevant'.
My original comment was @28. Please note that I was not happy with the 'layer' concept that others used-- it's not my invention. Also, I've said several times that I understand both the underlying physics concepts and the complexity of the problem. But I don't have a MODTRAN in my head, so I'm asking very specific questions to help me construct a model (or analogy) that is both reasonably accurate (for myself) and simple enough to use in conversations with those who are more lay than I am.
Since this is awfully wordy, I'm going to start a couple of new specific comments on the layer concept and the overall picture.
mgardner I am sorry that my posts have been interpreted as being adversarial or dismissive, they really were not intended that way, the intention was to try and warn that your questions were likely to lead down what appears to me to be a blind alley.
Note back on post 37 I tried to explain that a two layer model wasn't an accurate representation of reality, and by post 39 suggesting that it might be better to think of it in terms of a p.d.f. of the heights at which IR photons are successfully radiated. Note scaddenp's interesting diagram supports the idea that there isn't really an "opaque" layer at all, at least at current CO2 levels (which is why I was suggesting concentrating on the photons that actually escape the atmosphere).
Your use of terminology has not been all that clear, you write:
"... I clearly distinguish between "absorption of all possible photons from the emitting plane" (opacity, which is the essence of the original myth about 'saturation',) and "a reduction of the energy emitted from that plane as it traverses the interval" (attenuation). I don't use words randomly.
however your original question (repeated in that post) was
"And what the attenuation would be for IR radiation in some CO2 band through that 'layer'."
Specifying IR radiation in that band makes the use of attenuation ambiguous as the energy entering that region as IR in those bands that is absorbed is not necessarily transferred to the "translucent layer" as IR in those bands, and the IR that is emitted into space from the "translucent" layer largely comes from the bulk heat energy of the atmosphere at that height, which does not primarily come from IR in the absorption bands, but from convection. This means that the best definition of attenuation that works for this sentence as stated is in terms of attenuation of the photons themselves.
I'd be happy to resume the discussion on a non-adversarial, friendly tone, misunderstandings happen, especially in electronic forums!
DM@63
That pesky "real world" is intruding on our fun little universe here, so I will try to briefly give you something to think about while I take a little time to attend to it. I don't think we're quite communicating yet...
Here's what I mean when talking about an opaque layer. And please remember, I am setting up a simplified, idealized case to further my understanding-- I'm gathering up elements from which I can synthesize an accurate model that works for me, not anyone else. Once I do that, you are free to tell me that I am wrong or that yours is better.
Scaddenp's graph refers to emissions "from a hundred meter layer".
I treat that as a plane.
In my earlier reply to KR, I said something like:
"For some altitude h (e.g. 5 km), and for some wavelength (lambda), I think there is an altitude h-x such that the number of such (lambda) photons emitted from a plane at that level (h-k) which reach h is negligible."
and
"I don't know what x is, or how many subsequent such layers descending from h-x there may be."
To my mind, there are three legitimate ways to answer that:
1) "You're wrong, there's only one (or no such) layer and it goes to the surface. Here's the calculation using this wavelength and Beer's law." (KR's response, sans the calculation, which to me is obviously wrong.)
2) "You are correct, I come up with (n) such layers, and the thicknesses vary thusly as we descend."
3) "Sorry, I don't have the chops to provide you with the numbers you are looking for." or maybe "Sorry, you don't have the required security clearance?"
This can also be applied to the question of how the amount of radiant energy embodied in those photons is attenuated, as measured by radiant energy arriving at h.
Hope that's clearer. I will be back and try to discuss the more general case.
mgardner wrote "For some altitude h (e.g. 5 km), and for some wavelength (lambda), I think there is an altitude h-x such that the number of such (lambda) photons emitted from a plane at that level (h-k) which reach h is negligible."
My reading of scaddenp's graph suggests that for 400ppmv, the maximum power of photons in this band is emitted from about 4km in altitude (about 0.44 watts m^-2), but there is about 0.24 watts m^-2 of photons in this band that reach space directly from the surface, which is far from negligible. Thus there is no truely opaque layer (in this sense) from which the radiation of IR photons from the surface is negligible, at least for current atmospheric CO2 concentrations. Or to put it another way, whatever the values of h and x are, they are too large to fit within the Earths actual atmosphere.
I suspect you really need to use something like MODTRAN to get a definitive answer to this question, however the realclimate article I mentioned earlier in the thread provides this diagram:
Apparently at 300ppm, the CO2 in a column of the Earths atmosphere is equivalent to a 2.5m tube of pure CO2 (at sea level pressure and 20C). The graph shows the proportion of light transmitted through the tube as a function of length. In this case the transmission is still falling appreciably from tube lengths corresponding to 1xCO2 to 4XCO2, which suggests that there isn't a truely opaque layer within the tube in this thought experiment either. Note that this is not really realistic (as the article points out because not all of the atmosphere is at 20C or sea level pressure).
I'd say it was a mixture of (1) and (3), I don't (currently) have the chops to perform the calculations, but hopefully I can help in getting into the ball-park and help to better formulate the problem (you do have the required security clearance though ;o).
mgardner - I'm afraid you're mischaracterizing my posts (and perhaps mixing them with others, as I have not referenced Beers law). I've found your requests for 'opaque' layers quite difficult to decipher, in part as (a) there has been no definition of 'negligible' stated, (b) path length increases as a continuum with decreasing GHG concentration/pressure, and (c) you've kept asking about 'opaque layers' while stating that you think layers are not a proper way to think about the matter - the combination of those factors making it impossible to suggest any dividing lines. Or, quite frankly, for me to follow what you have been asking for. I have not intended to be rude - just trying to answer the questions as I understood them.
The discussion appears to be progressing now that there is sufficient understanding of terms and of your queries - I will leave you to that.
Dikran Marsupial @65, it is important to interpret scaddenp's graph in the light of KR's excellent annotated MODTRAN graph:
For context, Scaddenp's graph is for transmission of 13-17 micron radiation. That is radiation with a Wavenumber of 590-770 cm-1. As such it includes the full width of the notch shown in the graph above, not just the central values. A graph showing just 15 micron radiation (=666.7 cm-1) would show a much narrower effective emission profile for each concentration, and indeed, would show an altitude such that below that altitude, emitted IR radiation is nearly completely absorbed. In contrast, a graph of just 13 or 17 micron radiation would more closely resemble something like the 100 ppmv profile (I also do not have the chops to calculate it, and would need to do so to be sure).
Looking carefully at Scaddenp's graph, you will show that all curves have a right skew, so their mean value of emission is higher than their modal value. That makes things slightly awkward in that we need to guesstimate (if we cannot calculate) that mean value in relating it to the atmospheric profile shown by KR. However, in general the curve of a particular wave number in the 590-770 band will have an effective emission profile with a mean altitude of emission equivalent to the 1000 x (300- brightness temperature)/6.5. As such, even the emission profile of CO2 with a wave number equivalent to the bottom of the trough is not shown on Scaddenp's graph.
Quoting mgardner, he wrote:
As I have noted, at 15 microns (666.7 cm-1), that is correct. Indeed, at 15 microns absorption is so strong that I have seen a 15 micron spike in atmospheric spectra recorded in an aircraft from the amount of warmed, cabin air between the window and the instrument (much less than a meter). It is probably also true for the plateau in emissions on either side of 15 micron spike (which has a mean altitude of emission in the tropopause). However, it is not a usefull formulation for CO2 emission in general, because of necessity it will neglect the wings were the important action is occuring with respect to increases in CO2 concentration. On the other hand, in simplified models and discussions, climate scientists treat all emissions as coming from the mean altitude of emission - a simplification that is surprisingly accurate (I am told).
Cheers Tom, that is very interesting, it makes the shapes of scaddenp's plot a little less surprising (but still interesting), and hopefully helps answer mgardners question.
Tom Curtis - Credit for that excellent MODTRAN graph goes to HK, not to me. The handle are unfortunately easily cross-read.
At least this isn't as bad as when there were three different 'Hank's actively commenting...
Tom Curtis @67: Your point seems to agree with what I’ve tried to explain below, which was written before I read your last post:
Agree with Dikran that your diagram is very interesting! I’ve included it in my growing collection of climate graphics.
All of you: Regarding the discussion about of “opaque” layers, have you taken into account that the absorption of IR by CO2 is extremely dependent on the radiation’s wavelength, even within a relatively narrow wavelength interval like 13-17 microns in the diagram? I think that explains why the radiating layers of CO2seem to be so diffuse, i.e. spanning over so large altitude ranges.
Take a look at this diagram from the previously mentioned RealClimate article about the saturation argument. (It also demonstrates another important aspect of this, namely the widening of the absorption band.)
As you see, there is a more than 1000-fold increase of the absorption factor from 13 to 15 microns, and at least a 100-fold reduction from 15 to 17 microns. When that is taken into account, my interpretation of the diagram in @55 is the following:
The radiation from the lower troposphere (in the 1000 ppm case) comes from the periphery of the absorption band, say 13-13.5 and 16.5-17 microns. It’s weak despite coming from fairly warm CO2 because the radiation is partly blocked by CO2 higher up.
The radiation from the middle troposphere comes from wavelengths closer to the centre of the absorption band; say 13.5-14.5 and 15.5-16.5 microns. It’s stronger despite coming from colder CO2 because much less of the radiation is blocked by CO2 higher up. The radiation from the lower troposphere on these wavelengths is completely blocked because CO2 absorbs more strongly here.
The radiation from the upper troposphere and lower stratosphere comes from the central part of the absorption band (14.5-15.5 microns), with the radiation closest to 15 microns originating in the upper part of the stratosphere (even above Felix Baumgartner!). This radiation weakens partly because the CO2 here is very cold, and partly because the wavelength interval where CO2 is able to absorb and radiate gets narrower as the altitude increases (you get less radiation from a 1 micron wide interval than a 4 micron wide). And of course, it’s impossible for any photons in the 14.5-15.5 micron interval to escape to space from the lower and middle troposphere because CO2 absorbs so strongly in this part of the spectrum.
The same goes for the curves for lower CO2 concentrations, but the altitude with peak radiation (about 8 km for 1000 ppm) will be lower while the radiation itself is higher in the warmer parts of the atmosphere.
What if the diagram in @55 had showed radiation from a narrower wavelength interval, say 14.5-15.5 microns in stead of 13-17? I’m sure the radiation within each CO2 concentration would originate from a much narrower altitude interval and look more like a “layer” in the atmosphere (more like what mgardner has claimed).
Does all this make sense, or have I missed something crucial here?
[JH] Unnecessary white space eliminated.
KR @69, thanks for the advise. Oddly, I read "HK", and intended to type "HK", but apparently my fingers didn't get the message.
HK @70, first, apologies for the misattribution. Second, yes I believe we are saying the same thing.
I like the new diagram from RC. It is particularly interesting to pay attention to the logarithmic scale of the absorption factor, which should drive home the very large differences in the width of the emission profile for small changes in wavelength.
One slight point about the diagram, however. It includes the two relatively highly absorbing bands at 13.9 (720 cm-1) and 16.2 microns (620 cm-1)in the region saturated at 1xCO2. From the modtran graph, they are still clearly in the wings, and become deeper (less emissions to space) with higher CO2. The statement that the region is saturated is, therefore, clearly a simplification and should not in fact be understood as saying that there is no further strengthening of the greenhouse effect across that entire band with increased concentration.
Agree with your comment about the non-saturation, Tom!
Unlike the MODTRAN graph, the annotations in the diagram were not added by me. They can be found in the original diagram in the article from 2007.
HK@70
"What if the diagram in @55 had showed radiation from a narrower wavelength interval, say 14.5-15.5 microns in stead of 13-17? I’m sure the radiation within each CO2 concentration would originate from a much narrower altitude interval and look more like a “layer” in the atmosphere (more like what mgardner has claimed). "
HK, please look at the graph @55. Look at the label of the vertical axis.
Now, read carefully what I said @64.
What have I so far claimed about radiation originating in a 'layer' other than what the graph says? Again, my original problem was the original 'layers' language of the explanation-- about 'radiating layers moving up to where it is colder'.
I've now quoted myself so often I guess there is not much point-- I think it is the hammer and nail problem. Whatever question I ask, the answer is going to be the one that everyone has learned to repeat.
[JH] Ypou are now skating on the thin ice of escessive repetition which is prohibited by the SkS Comments Policy. Please cease and desist.
mgardner, as you continue to be ill-mannered and dismissive towards attempts to address your ill-posed questions, even after my appology in post 63, consider my participation in this discussion over.
mgardner,
The people you are posting with are volunteers who have gone to a lot of effort to find graphs and explainations that will answer your questions. They are searching the background data for information outside of their specialties. You have thanked their efforts with criticism. Has it occured to you that the problem may be that your questions have been poorly phrased from the beginning? You appear to have specific needs for your answer that it is difficult for others to understand. Perhaps you need to do your own homework, instead of criticizing others for doing a poor job of it. You could be building your knowledge by searching for the answer yourself.
mgardner @64 & 73:
You want some examples of how long a path of air must be to reduce the amount of IR radiation passing from one end to the other to a “negligible” level?
If a “negligible” fraction of surviving radiation is defined as 1% (my arbitrary choice), it should be possible to calculate some path lengths of air needed to achieve this, assuming that my layman understanding of the Beer-Lambert law is reasonably correct. First a hypothetical example to show the principle:
Suppose that 25% of the radiation at a particular wavelength is absorbed by 1 metre of air. The next metre will absorb 25% of the remaining radiation and the same fraction will be absorbed by each successive metre after that (75% surviving through each metre).

The surviving fraction through 5 metres is 0.755 = 0.237, for 10 metres the fraction is 0.7510 = 0.056 and so on. In this case it takes 16 metres of air to reduce the surviving radiation to a negligible level, since 0.7516 = 0.01 = 1%.
And now some real data:
This graph from this article by Science of Doom shows the fraction of radiation entering one end of a 1 metre path of air at sea level that is transmitted (survives) to the other side. The CO2 concentration is 380 ppm, and the radiation originating within the air itself is ignored. The y-axis goes from 0 (no radiation transmitted) to 1 (all radiation transmitted), and the x-axis covers the wavelength interval from 15.15 micron (left) to 14.88 micron (right), i.e. around the very strongest absorption band of CO2. (10,000 / wavenumber = wavelength in microns)
As you see, 99% of the radiation is transmitted at 662 cm-1, while only 2% is transmitted at 667.5 cm-1, so the radiation at 667.5 cm-1 is already reduced to an almost negligible level by only one metre of air at sea level!
A calculation like the one above shows that it takes 458 metres of air to reduce the radiation at 662 cm-1 to a negligible level (0.99458 = 0.01) while it only takes 1.18 metres (0.021.18 = 0.01) to achieve the same for radiation at 667.5 cm-1. (See the last paragraph in Tom’s comment @67)
If we ignore the complexities caused by pressure broadening and so on (I’ll leave that to the experts!), these path lengths are roughly inverse proportional to the atmospheric pressure. If the pressure drops to 0.2 bar (at about 12 km) the number of CO2 molecules per m3 is only 1/5 of that at sea level, so you need a 5 times longer path to get the same absorption as at sea level. That should translate to 2.3 km for radiation at 662 cm-1 but still only 5.9 metres for radiation at 667.5 cm-1. In the middle of the stratosphere (30 km / 0.01 bar) the same path lengths are about 46 km (!) and 118 metre, respectively.
(this approach only works for well-mixed gases like CO2, not for water vapour that is strongly concentrated in the lower troposphere)
So, mgardner, the “x” you have been asking for can be anything from a metre to tens of kilometres, depending on wavelength, altitude and other factors, including how you define the term “negligible”.
Did this help?
HK@76
Thank you. Like your other posts, this is very thorough and well written.
My interest is in the area (nominally 5 km) where we say that 'most of the energy is radiated to space'. And, because my goal is to explain or 'draw a picture' (literally or verbally) at the simplest level, I wish to discuss only one wavelength resulting in somewhere between big x and little x.
So, let's say we define at about 5 km two layers of 100 m thickness (as in that graph @55 we both like). We call the top one A and just below it B. (I use top and bottom "plane" to indicate the boundaries not to confuse with "the" surface of Earth). Also, I will simply use zero rather than negligible for quantities. And, CO2 is the only GHG for this discussion.
A has zero CO2, and B has some. (I say that A is transparent, and B is translucent.) C, which begins at the bottom plane of B and goes down x, is 'opaque' since nothing originating below its bottom plane reaches B.
How do we characterize the radiation flux and the temperature for these layers, first as described, and then after we add CO2 in the equivalent amounts to each?
mgardner @77, your question serves no apparent purpose.
You have asked a series of questions on this topic, and have had a perfectly acceptable response to those questions since at least Dikran Marsupial's responce @37, where his final paragraph stated:
Nothing since then has done any more than flesh out details of this basic picture, and perhaps illustrate it better. One key point here is that talking in terms of layers as hard boundaries defined for x,y, such that below x, at wavelength y, no photons emitted escape to space makes no sense. That is DM's first point in the paragraph above. And yet you return to the assumption that such defined boundaries make sense, that in scientific use they are anything more than an approximate, but sometimes useful convention.
In radiation models, the atmosphere is divided into thin layers. For each layer, l, and for each wave number (or some other conveniently small division of frequency) emissions are calculated. The absorption of the total upward radiation for the layer below, l-1, is calculated. The model then passes to the next upward, l+1, layer the total upward radiation from l, ie, the sum of upward emissions plus (1-absorption) of the total upward radiation from the preceding layer. The same is done for downward radiation in the opposite direction. No attempt it made to track the exact point of origin of radiation in the total upward radiation from each level because it is superfluous information. Such models calculate the actual upward or downward radiation at any level with stunning accuracy.
If asked where the radiation that escapes to space comes from, they modellers indicated that it comes from the "effective altitude of radiation", which is defined as "that altitude that has the same temperature as the brightness temperature of the radiation to space at a given wave number" (or other conveniently small division of frequency). Thus talk of the layer from which radiation comes is just a shorthand way of mentioning the temperature weighted mean altitude from which the radiation comes. Nothing more.
In more popular parlance, we can say (and this is literally true) that increased CO2 increases the mean altitude from which the IR radiation escapes to space, and that if that mean altitude is cooler, then the IR radiation escaping to space will be reduced. But the further details for which you seek serve no purpose either scientifically, or in terms of general education.
Tom Curtis - Agreed. I believe I stated one of those points before regarding the range of altitudes emitting, "...the sum of that emission adding up to the oft-noted temperature equivalent profiles seen in satellite observations of those band". This makes emission from a single layer at that observed temperature an incorrect way of thinking about the physics, as it is a mean altitude, not a single one.
Tom Curtis@78
"But the further details for which you seek serve no purpose either scientifically, or in terms of general education."
You are welcome to your opinion, Tom.
With respect to general education, I have always assumed that I was protected by the concept of academic freedom in making those kinds of decisions for myself. I do have some experience in this area, and, unless you plan to fly in for the next holiday, you are not the one who is going to be drawing little pictures for my brother-in-law, right?
With respect to scientific purpose, some of the most fruitful physicists of our time have "gone back to the classroom"-the physics 101 classroom-- because it served to clarify their conceptualizations. At the least, for an interested student, wouldn't these 'purposeless' inquiries give some insight into the thinking of those who developed MODTRAN, to which we refer so often?
And then there's the final question-- what's the harm? If HK wants to exercise his chops, is there some policy that prohibits it? I was accused of being 'critical' but several people seem to want to criticize my curiosity. I have always been delighted (and amazed) when students thought for themselves and asked questions.
If this gets me moderated out, so be it. You seem to be much more welcoming and tolerant of 'skeptics' who take up lots of space to little effect.
[JH] Please lose the condescending attitude.
mgardner @80, you are welcome to your opinion, and to waste your time pursuing it. You are not welcome to waste my time on trivial technicalities. Look it up and work it out for yourself. Then when you have, if you still think it interesting, come and let us know the answer, and why it is interesting. In the meantime, your repeated questioning on a trivial point is just spam. Don't be surprised, therefore, if we put you in our personal spam folders (ie, ignore what you have to say, and ask questions about) in future.
One addition to my post @81, mgardner has been quite critical of people above for not answering the questions he purportedly asked. He has acted, in other words, as though they had an obligation an answer him. Well, they do not. They do not, firstly because they are volunteers; secondly, because he is ungrateful; and thirdly because the question he wants to focus on is uninteresting. There are far more scientifically informative questions that could be asked - like for example, "how does modtran work". When provided with answers to those informative questions (ie, informative answers to his questions as posed), mgardner has been at his most ungrateful.
He will now, no doubt, complain that his ingratitude has lead to his questions being ignored. He is welcome to those complaints to. He is just not welcome to anymore of my time.
This could be a case of "I don't (can't, won't) understand until I get the result that I'm looking for."
I want to lift your spirits guys by saying that someone like me, has been reading your explanations and learned, as opposed to mgardner, a lot from your posts. Thank you! Especially to HK for very informative pictures and reference to Science of Doom, to Bob Loblaw for reference to Manabe 1964, and to Tom Curtis for overall summary. Sorry to those I did not manage to mention this time...
Like mgardner, I can boast that I have the necessary backgroound to grasp this science, but my attitude, unlike mgardner's, respect the science first (and all people who made it possible for me to learn it, starting from Manabe-san in the topic at hand), then try to assimilate it, finally comment/ask questions only at the end when new knowledge is too difficult or incompatible with my previous knowledge. mgardner's priorities are backwards to my priorities.
Tom@79, your explanation what radiation models do can be summarised as: double integration of IR energy function along altitude and frequency. If the function was as simple as Beer-Lambert law (also applicable to pressure vs altitude) and each frequency were absorbed independently, we'd have an easy analytical solution. But because the world is not that simple (natural broadening due to Heisenberg principle, Doppler broadening due to fast moving - 500m/s - air molecules, finally pressure, or collisional, broadening due to collisions between CO2 and other air molecules) the function becomes too complex, and frequencies inter-dependent to resolve it analytically. Therefore, it is resolved numerically as you described. The "effective altitude of radiation" as a is such model is an abstract term, defined by the amount of energy caried out to space within a particular band of IR and the lapse rate. That's a nice, valid definition and makes perfect intuitive sense to me [1]... Further, if you integrate total IR, energy you can define the "mean altitude of radiation" as total amount of IR energy within the lapse rate. And again, it makes perfect intuitive sense.
With such definition, the argument in the subject article (that GHE never saturates) can be understood even better. If you add CO2, the mean altitude of radiation will always rise no matter how much CO2 you had in the first place (even on Venus). That's because:
- the initial mean altitude of radiation cannot be infinite (some energy must be escaping the planet, otherwise the planet would heat up infinitely which is absurd)
- the aditional CO2 must be pushing the initial mean altitude higher, because all of the physical phenomenons involved (gas mixing, Beer-Lambert law, Doppler broadening, pressure broadening) collectively increase the IR absorption at the previous mean altitude of radiation.
------------------
[1] Well, except the case of a band around 667cm-1, which is emitted from stratosphere: it could have been emitted from troposphere at the same temperature (linear T lapse rate does not hold in stratosphere) so there is an ambiguity in this case.
I just found this excellent blog post by Clive Best about the forcing from a doubling of CO2 based on how much that will change the "effective emission height", which is another term for "altitude of heat loss". It covers much of the topic we've been discussing here, and maybe it can fill some of the holes in our understanding.
HK @85, first a word of caution. Clive Best is an AGW 'skeptic', and while he is more mathematically sophisticated than most AGW 'skeptics', he still breathlessly writes about the lack of warming over the last twelve years, and predicts cooling temperatures for the next decade because the lower uncertainty bound of the HadCM2 model short term climate forecast permits it. Any recommendation of one blog post by Clive Best should not be construed as a recommendation of any other blog post by Best, or the quality of his blog in general.
More importantly, Clive Best's attempt to calculate the effective altitude of radiation clearly fails on empirical grounds. Specifically, this is his calculated "effective altitude of radiation":
Clearly he shows the effective altitude of radiation on either sides of the spikes at 620 and 720 cm-1 as being between zero and 1000 meters. In contrast, as can be seen in the real spectrum he shows, at those wave numbers, the effective altitude of radiation is closer to 6000 meters {calculated as (ground temperature - brightness temperature)/lapse rate}:
As can be seen from his graph of the predicted IR spectra, he clearly gets the 660 cm-1 spike wrong as well, showing it as a dip (?!) for 300 ppmv, and as a barely discernable spike at 600 ppmv. That is so different from the obvious spike in the real world spectrum (at approx 390 ppmv) that you know (and he should have known) that he has got something significantly wrong.
Before addressing that specifically, I will note two minor things he omitted (perhaps for simplicity). The first is that he has not included a number of factors that broaden the absorption lines. Broadening increases the width of the lines, but also reduces the peak absorbance of the lines. In any event, he has not included doppler broadening, possibly does not include collissional broadening, and probably does not include some of the other minor forms of broadening.
The second factor is that he has not allowed for the difference in atmospheric profiles between the US Standard atmosphere and actual tropical conditions. Specifically, the atmosphere is thicker at the equator due to centrifugal "force", and also has a higher tropopause due to the greater strength of convective circulation. That later should reduce CO2 density, and might be accepted as the cause of the discrepancy except that mid latitude and even polar spectra show the same reduce absorbance relative to his calculated values (and hence higher effective altitude of radiation in the wings, and for the central spike).
Although these factors are sources of inaccuracy, they do not account for the major error in calculation. That is probably a product of his definition of effective altitude of radiation, which he defines as the highest altitude at which "... the absorption of photons of that wave length within a 100m thick slice of the atmosphere becomes greater than the transmission of photons". That is, it is the altitude of the highest layer at which less than half of the upward IR flux at the top of that altitude comes from that layer.
This definition is superficially similar to another common definition, ie, the lowest altitude from which at least half of the photons emitted upward from that altitude reach space. Importantly, however, this later definition is determined by the integrated absorption of all layers above the defined layer. Specifically, it is the layer such that the integrated absorption of all layers above it = 0.5. I think the layer picked out by Best's method is consistently biased low relative to that picked out by this later definition.
There are two other common definitions of the effective layer of radiation around. The most common is:
(Source, h/t to Science of Doom)
That definition can be generalized to specific wave numbers, or spectral lines, and is used by Best in an earlier blog post specifically on the subject. It also needs to be modified slightly to allow for the central spike (which comes from the stratosphere). The difficulty of such a modification, plus a certain circularity in this definition makes others preferable. The third definition is the one I give above of "the temperature weighted mean altitude from which the radiation comes". I take it that the three common definitions pick out the same altitude, at least to a first order approximation. In contrast, Best's definition in the blog post to which you refer is of by (in some portions of the spectrum) at least 6 kms.
Despite this flaw, Best's blog post does give a good idea of the methods used in radiative models. However, his detailed results are inaccurate, in a way that does not reflect the inaccuracy of the radiative models used by scientists. This also applies to the graph shown by scaddenp @55 above, which was also created by Best. It is very indicative of the type of profiles likely to be seen, but should not be considered an accurate source. I discuss the accuracy of actual models briefly here, and in more detail in the comments.
Thanks for the information and warning, Tom!
I didn’t study Best’s graph very methodically, just noted that at a first glance it more or less resembled the results from the Nimbus satellite and the MODTRAN model. He even estimated the forcing from doubled CO2 to be a little higher than the most commonly stated 3.7 watt/m2.
It’s quite puzzling to me that someone can be an AGW skeptic (I prefer the word “denier” if they reject well-understood science) when they seem to understand the basic physics about how the greenhouse effect works by rising the altitude of heat loss, however that term is defined.
The argument “no warming over the last 12 years” in particular seems quite amateurish when Best should understand the insignificance of short periods, that only 1% of the heat accumulation happens in the atmosphere and that the alleged “hiatus” of surface warming maybe doesn’t even exist. The same applies to the argument of near-term cooling because the uncertainty of a climate model permits it. I’m not a statistician and don’t grasp the more technical aspects of Tamino’s usually excellent posts, but I do understand the simple concept that uncertainty goes both ways. It’s kind of understandable if a wrong conclusion is the result of wrong data, but it’s worse if a wrong conclusion is based on the correct data and a faulty logic.
Again, thanks for the information, Tom!
HK (#76):
I don't know if this was covered earlier on; but you need to be careful about trying to apply Beer's law to thermal radiation.
Beer's law describes an exponential dying away of intensity as radiation progresses. But thermal radiation does not fritter away to zilch, it fritters away until it reaches the intensity of radiation appropriate for the frequency and the temperature of the gas through which it is passing.
If thermal radiation were simply dissipated by passing through a medium, visible light would never escape the Sun. Visible light is the thermal radiation in the Sun, just as IR is the thermal radiation in the atmosphere.
The subject to look into regarding this is "radiative transfer".
I’m aware of that, nealjking. My calculations only considered transmittance of the original radiation entering the path of air, not re-emission within the air itself. That, combined with the cooling and thinning air with altitude, is a crucial part of the non-saturation argument, as Glenn Tamblyn pointed out in the blog post.
If the only radiation escaping to space came directly from the surface while the atmosphere only absorbed without re-emitting, much of the radiation shown in the MODTRAN graph in @67 would be virtually zero.
I thought this might be of interest; I hope it can be posted:
NY Times Article on Communication
Alan Alda on his work for Scientific American
Over the years, I must have done around 700 of these interviews, and I felt that in doing them I had stumbled onto something that could help solve a big problem the science community faces.
Which is?
That scientists often don’t speak to the rest of us the way they would if we were standing there full of curiosity. They sometimes spray information at us without making that contact that I think is crucial. If a scientist doesn’t have someone next to them, drawing them out, they can easily go into lecture mode. There can be a lot of insider’s jargon.
If they can’t make clear what their work involves, the public will resist advances. They won’t fund science. How are scientists going to get money from policy makers, if our leaders and legislators can’t understand what they do?
I heard from one member of Congress that at a meeting with scientists, the members were passing notes to one another: “Do you know what this guy is saying?” “No, do you?”
[PS] This is offtopic. I would appreciate if you would repost here. Thank you.
mgardner:
I would recommend re-posting this comment on one of the Weekly Digest or Weekly Round-Up threads, which being more-or-less "open threads" are topical for just the sort of thing you have posted, while it is off-topic for this thread.
scaddenp:
Where did you get the graph shown at #55 ?
nealjking @92, I don't know where scaddenp got it, but it was constructed by Clive Best. Best used his own radiation model for which he provides code. There was also a spreadsheet based implimentation of an earlier version, but the link is dead. The model only calculates values in the 13-17 micron range, and treats it as a single band. That means the results are approximate rather than accurate but still indicative.
I did get it from Clive Best (just examine the link). I am aware of Best's opinions but at a quick look, it was the diagram I was looking for more or less in online form.
"So if we add more greenhouse gases the air needs to be thinner before heat radiation is able to escape to space"
But only for heat radiation absorbed and emitted by CO2. Heat radiation from the rest of the atmosphere is at the same altitude as before, since we arent adding more N2, O2 etc.
So even if this theory is true, 0.004% of the atmopshere radiates from a higher altitude. 99.996% radiates from exactly the same altitude as before.
Therefore the saturation argument still holds true and increasing CO2 is of little effect.
[PS] Friendly advice. If every textbook on radiative physics validates GHG theory, but you have a differing opinion, then chances are you have misunderstood the theory (as in this case) rather than the theory is wrong. Concluding "therefore the saturation argument still holds" is hubris in extreme. You will get better engagement here if instead you say "given x,y,z, then appears to me that the theory X is wrong". People will help with misconceptions and in the unlikely event of you discovering something new, be inclined to take your argument seriously. And you are very unlikely to find new science unless you have taken time to read the textbook and thoroughly understand the theoretical background.
ConcernedCitizen @95, only a few atmospheric gases radiate heat in the IR spectrum. In particular, O2 radiates in the microwave range where very little energy is emitted, and the visible light range where no energy in emitted at atmospheric temperatures. As a result radiation from O2 is inconsequential. In a similar manner, radiation from N2 is even more inconsequential. Of the major IR radiating gases (CO2, CH4, NO2, O3) CO2 has a far greater abundance than the others, and absolutely dominates their effect. Further, the only of the IR gases to be more abundant than CO2 at low levels (H2O) precipitates out with increasing altitude, and consequently is far less abundant than CO2 at high levels.
The upshot is that your reasoning is fatally flawed by reason of radically false premises.
All of this is largely irrelevant, however. The theory used to predict the impact of increasing CO2 has been used to program radiation models that show stunning accuracy in predicting the observed IR radiation from the planet. This, for example, is the type of accuracy that they demonstrated in 1970 (46 years ago):
The large trough centered around 15 micrometers wavelength is, of course, due to CO2. Area under the curve represents the total power of TOA emissions, so that trough represents a very significant reduction in energy radiating to space.
Similar observations have been made with similar accuracy across a wide range of atmospheric condition. For example, in 2008 comparisons between a model and satellite observations for 134,862 measured values were released:
This represents a stunning accuracy, and the fact that the accuracy was preserved over the full range of latitudes, surface types and atmospheric temperatures shows it is no accident.
Against this very well established theory - confirmed by laboratory and in situ observations to a remarkable extent, you offer hand waving based on radically false premises. Given that you have no model, ie, no mathemtical predictions of observations from your premises, you do not even have a scientific theory. But you want your hand wavey non-theory to trump a theory backed by detailed and extensive comparisons between models and observations over nearly half a century. I'm just not buying it.
@Moderator. Where did I question the GH theory?
I am questioning the 'more CO2 = higher lapse rate thus more surface warmig' theory you are proposing in this piece.
@96. You misunderstand. The suggestion that the atmosphere is made thicker by adding CO2, thus forcing it to radiate from a higher altitude and causing surface warmuing would have to be acompanied by an increase in pressure at the surface,. This hasnt happened.
ConcernedCitizen @98:
Neither you nor the OP said anything like this, so your claim that it did is simply false.
As it happens, the addition of CO2 to the atmosphere does not make it thicker (each molecule of CO2 replaces a molecule of O2, but as CO2 dissolves more readilly in water, the total number of molecules in the atmosphere decreases by a miniscule amount). Because CO2 is heavier than O2, overall the mass of the atmosphere has increased by about 256 Gigatonnes (0.005%) due to increased CO2 , but fallen by 558 Gigatonnes due to O2 being converted to CO2 then dissolving in the ocean or being taken up by plants. The net reduction is approximately 302 Gigatonnes, or about 0.006%. That is negligible and well below the impact of increased humidity on atmospheric mass.
Finally, overall atmospheric pressure is predicted to increase due to increased temperature - and is increasing to a greater extent than predicted by models so your final claim is also false. It has no relevance to the greenhouse effect other than as a predicted response to warming.
Your completely false claims, coupled with the egregious way in which they misrepresent the OP has taken us way of topic so I will not respond to further egregious misrepresentations other than to note that they are in fact egregiously false, and misrepresentations.
If no one can explain why CO2 is radiating from a higher lattitude then can we assume than that it isnt and that the article is false?