Is the CO2 effect saturated?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
The notion that the CO2 effect is 'saturated' is based on a misunderstanding of how the greenhouse effect works. |
|||||||
Climate Myth...
CO2 effect is saturated
"Each unit of CO2 you put into the atmosphere has less and less of a warming impact. Once the atmosphere reaches a saturation point, additional input of CO2 will not really have any major impact. It's like putting insulation in your attic. They give a recommended amount and after that you can stack the insulation up to the roof and it's going to have no impact." (Marc Morano, as quoted by Steve Eliot)
At-a-Glance
This myth relies on the use (or in fact misuse) of a particular word – 'saturated'. When someone comes in from a prolonged downpour, they may well exclaim that they are saturated. They cannot imagine being any wetter. That's casual usage, though.
In science, 'saturated' is a strictly-defined term. For example, in a saturated salt solution, no more salt will dissolve, period. But what's that got to do with heat transfer in Earth's atmosphere? Let's take a look.
Heat-trapping by CO2 in the atmosphere happens because it has the ability to absorb and pass on infra-red radiation – it is a 'greenhouse gas'. Infra-red is just one part of the electromagnetic spectrum, divided by physicists into a series of bands. From the low-frequency end of the spectrum upwards, the bands are as follows: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Gamma rays thus have a very high-frequency. They are the highest-energy form of radiation.
As our understanding of the electromagnetic spectrum developed, it was realised that the radiation consists of particles called 'photons', travelling in waves. The term was coined in 1926 by the celebrated physicist Gilbert Lewis (1875-1946). A photon's energy is related to its wavelength. The shorter the wavelength, the higher the energy, so that the very high-energy gamma-rays have the shortest wavelength of the lot.
Sunshine consists mostly of ultraviolet, visible light and infra-red photons. Objects warmed by the sun then re-emit energy photons at infra-red wavelengths. Like other greenhouse gases, CO2 has the ability to absorb infra-red photons. But CO2 is unlike a mop, which has to be wrung out regularly in order for it to continue working. CO2 molecules do not get filled up with infra-red photons. Not only do they emit their own infra-red photons, but also they are constantly colliding with neighbouring molecules in the air. The constant collisions are important. Every time they happen, energy is shared out between the colliding molecules.
Through those emissions and collisions, CO2 molecules constantly warm their surroundings. This goes on all the time and at all levels in the atmosphere. You cannot say, “CO2 is saturated because the surface-emitted IR is rapidly absorbed”, because you need to take into account the whole atmosphere and its constant, ongoing energy-exchange processes. That means taking into account all absorption, all re-emission, all collisions, all heating and cooling and all eventual loss to space, at all levels.
If the amount of radiation lost to space is equal to the amount coming in from the Sun, Earth is said to be in energy balance. But if the strength of the greenhouse effect is increased, the amount of energy escaping falls behind the amount that is incoming. Earth is then said to be in an energy imbalance and the climate heats up. Double the CO2 concentration and you get a few degrees of warming: double it again and you get a few more and on and on it goes. There is no room for complacency here. By the time just one doubling has occurred, the planet would already be unrecognisable. The insulation analogy in the myth is misleading because it over-simplifies what happens in the atmosphere.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
This myth relies on the use of a word – saturated. When we think of saturated in everyday use, the term 'soggy' comes to mind. This is a good example of a word that has one meaning in common parlance but another very specific one when thinking about atmospheric physics. Other such words come to mind too. Absorb and emit are two good examples relevant to this topic and we’ll discuss how they relate to atmospheric processes below.
First things first. The effect of CO2 in the atmosphere is due to its influence on the transport of 'electromagnetic radiation' (EMR). EMR is energy that is moving as x-rays, ultraviolet (UV) light, visible light, infrared (IR) radiation and so on (fig. 1). Radiation is unusual in the sense that it contains energy but it is also always moving, at the speed of light, so it is also a form of transport. Radiation is also unusual in that it has properties of particles but also travels with the properties of waves, so we talk about its wavelength.
The particles making up radiation are known as photons. Each photon contains a specific amount of energy, and that is related to its wavelength. High energy photons have short wavelengths, and low energy photons have longer wavelengths. In climate, we are interested in two main radiation categories - firstly the visible light plus UV and minor IR that together make up sunshine, and secondly the IR from the earth-atmosphere system.
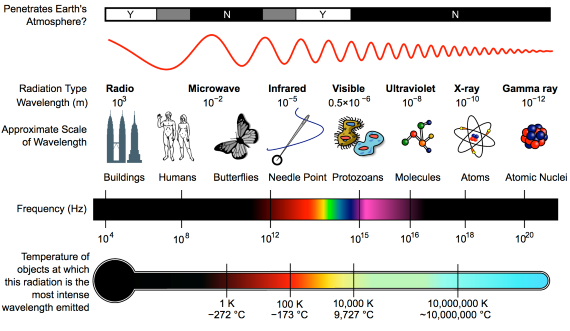
Fig. 1: diagram showing the full electromagnetic spectrum and its properties of the different bands. Image: CC BY-SA 3.0 from Wikimedia.
CO2 has the ability to absorb IR photons – it is a 'greenhouse gas'.So what does “absorb” mean, when talking about radiation? We are all familiar with using a sponge to mop up a water spill. The sponge will only absorb so much and will not absorb any more unless it's wrung out. In everyday language it may be described, without measurements, as 'saturated'. In this household example, 'absorb' basically means 'soak up' and 'saturated' simply means 'full to capacity'. Scientific terms are, in contrast, strictly defined.
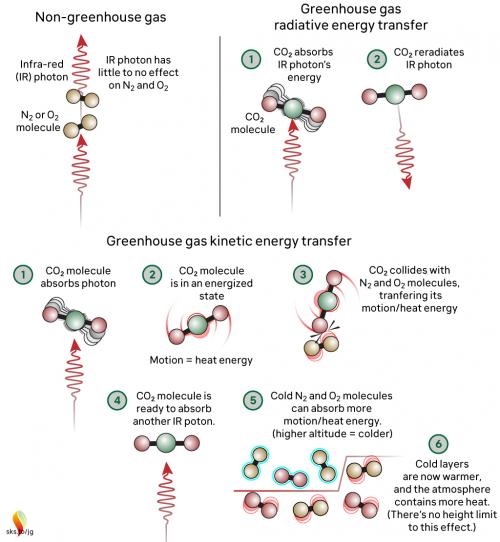
Now let's look at the atmosphere. The greenhouse effect works like this: energy arrives from the sun in the form of visible light and ultraviolet radiation. A proportion reaches and warms Earth's surface. Earth then emits the energy in the form of photons of IR radiation.
Greenhouse gases in the atmosphere, such as CO2 molecules, absorb some of this IR radiation, then re-emit it in all directions - including back to Earth's surface. The CO2 molecule does not fill up with IR photons, running out of space for any more. Instead, the CO2 molecule absorbs the energy from the IR photon and the photon ceases to be. The CO2 molecule now contains more energy, but that is transient since the molecule emits its own IR photons. Not only that: it's constantly colliding with other molecules such as N2 and O2 in the surrounding air. In those collisions, that excess energy is shared with them. This energy-sharing causes the nearby air to heat up (fig. 2).
Fig. 2: The greenhouse effect in action, showing the interactions between molecules. The interactions happen at all levels of the atmosphere and are constantly ongoing. Graphic: jg.
The capacity for CO2 to absorb photons is almost limitless. The CO2 molecule can also receive energy from collisions with other molecules, and it can lose energy by emitting IR radiation. When a photon is emitted, we’re not bringing a photon out of storage - we are bringing energy out of storage and turning it into a photon, travelling away at the speed of light. So CO2 is constantly absorbing IR radiation, constantly emitting IR radiation and constantly sharing energy with the surrounding air molecules. To understand the role of CO2, we need to consider all these forms of energy storage and transport.
So, where does 'saturation' get used in climate change contrarianism? The most common way they try to frame things is to claim that IR emitted from the surface, in the wavelengths where CO2 absorbs, is all absorbed fairly close to the surface. Therefore, the story continues, adding more CO2 can’t make any more difference. This is inaccurate through omission, because either innocently or deliberately, it ignores the rest of the picture, where energy is constantly being exchanged with other molecules by collisions and CO2 is constantly emitting IR radiation. This means that there is always IR radiation being emitted upwards by CO2 at all levels in the atmosphere. It might not have originated from the surface, but IR radiation is still present in the wavelengths that CO2 absorbs and emits. When emitted in the upper atmosphere, it can and will be lost to space.
When you include all the energy transfers related to the CO2 absorption of IR radiation – the transfer to other molecules, the emission, and both the upward and downward energy fluxes at all altitudes - then we find that adding CO2 to our current atmosphere acts to inhibit the transfer of radiative energy throughout that atmosphere and, ultimately, into space. This will lead to additional warming until the amount of energy being lost to space matches what is being received. This is precisely what is happening.
The myth reproduced at the top – incorrectly stating an analogy with roof insulation in that each unit has less of an effect - is misleading. Doubling CO2 from 280 ppm to 560 ppm will cause a few degrees of warming. Doubling again (560 to 1130 ppm) will cause a similar amount of additional warming, and so on. Many doublings later there may be a point where adding more CO2 has little effect, but recent work has cast serious doubt on that (He et al. 2023). But we are a long, long way from reaching that point and in any case we do not want to go anywhere near it! One doubling will be serious enough.
Finally, directly observing the specific, global radiative forcing caused by well-mixed greenhouse gases has - to date - proven elusive. This is because of irregular, uncalibrated or limited areal measurements. But very recently, results have been published regarding the deep reinterrogation of years of data (2003-2021) from the Atmospheric Infrared Sounder (AIRS) instrument on NASA's Aqua Satellite (Raghuraman et al. 2023). The work may well have finally cracked the long-standing issue of how to make finely detailed, consistent wavelength-specific measurements of outgoing long-wave radiation from Earth into space. As such, it has opened the way to direct monitoring of the radiative impact (i.e. forcing + feedback) of greenhouse gas concentration changes, thereby complimenting the Keeling Curve - the longstanding dataset of measured CO2 concentrations, down at the planet's surface.
Note: Several people in addition to John Mason were involved with updating this basic level rebuttal, namely Bob Loblaw, Ken Rice and John Garrett (jg).
Last updated on 31 December 2023 by John Mason. View Archives































 Arguments
Arguments





































Bob Loblaw @775
Sure! Try A First Course in Atmospheric Radiation, 2nd edition, by Grant W. Petty. He first gets into Beer's Law at page 78. I should warn you, however, this book does assume that you already recognize that it is perfectly legal to consider the EMR one frequency at a time since we are dealing strictly with linear equations. If this gives you heartburn, I might suggest you first study some basic E&M and differential equations.
I should also point out that I am not using any tricks that are not already used by you and your AGW believing comrades.
Bob Loblaw @775
Sure! Try A First Course in Atmospheric Radiation, 2nd edition, by Grant W. Petty. He first gets into Beer's Law at page 78. I should warn you, however, that this book assumes you already know that since we are dealing strictly with linear optics, it is perfectly legitimate to break down the total EMR into individual frequencies, and consider each one as independent of the others. If this gives you heartburn, then I suggest you study up on some basic E&M.
I should also point out that I am not using any tricks not already used by your AGW believing comrades.
Congratulations, CallItAsItIs. You actually have some sort of access to a textbook that covers "radiometery", and you know how to look in an index.
I am familiar with Petty's book, although It is not one that I have on my personal bookshelves.
I'll see your Petty, and raise you a Wallace and Hobbs, "Atmospheric Science, an Introductory Survey" (Beer's Law discussed on pages 296-297), a Pierrehumbert "Principles of Planetary Climate" (Beer's Law discussed in chapters 4 and 5), a Liou "An Introduction to Atmospheric Radiation" (which has 6 sections listed in the index for the Beer-Bouguer-Lambert law), and an Oke "Boundary Layer Climates" (also multiple references in the index).
All of those four books are ones that I do have on my personal bookshelf.
And if you want to see what else I know about Beer's Law, you can read this post:
https://skepticalscience.com/from-email-bag-beer-lambert.html
Yes, Beer's Law applies to individual wavelengths/frequencies. I challenge you to find a single reference that supports your argument that "conservation of energy must hold for each frequency independently of the others."
You see, Beer's Law says nothing at all as to what happens to energy that is absorbed when photons disappear within the volume of air it includes. As far as Beer's Law is concerned, the energy simply disappears along with the photon. To apply "conservation of energy" principles, you need to include where that energy goes - which you repeatedly fail to do.
CallItAsItIs @776 and @777 :-
Curiouser and curiouser . . . you make two almost identical posts, just 16 minutes apart. Can I believe the evidence of my "linear optics"? (Please excuse my humorously lame misquote from a certain third party.)
And in post @778, you see another "Law" (i.e. the LobLaw of Unintended Consequences ).
Also, CallItAsItIs, you state (twice) that: "I should also point out I am not using any tricks not already used by your AGW believing comrades." [unquote, unquote]
And there we touch upon the heart of your problem. Motivated reasoning. Motivated Reasoning does produce ~ even in an intelligent person such as yourself ~ the most remarkable contortions of self-contradictory assertions. As we have seen.
CallItAsItIs, you have a great deal of your own statements to reconcile. In the meantime, I shall get me some more popcorn, and a comfortable chair.
Bob and Eclectic
Callitasitis strongly reminds me of a user who has been banned repeatedly. I doubt that you will be able to explain thermodynamics to them no matter what you post.
Keep in mind that the comments policy doesn't allow repetition. When Callitasitis repeats themselves repeatedly it is time to let other readers decide who has presented the better arguments.
Eclectic @779
First, I would like to let you know that my posting very nearly the same comments twice was unintentional. I submitted the first one but it didn't show up on the webpage. So, I figured that somehow it got lost and I rewrote it and re-posted it. But it seemed to get "lost" again. Finally, I realized that I had started a new page with my new comment. So please disregard one of those postings and your moderator is welcome to remove it.
Next, the reason I "failed" to include where the absorbed photon energy goes is because I thought we already knew that. Primarily, it is distributed as kinetic energy to the atmospheric gases through collisions of excited CO2 molecules with N2 or O2. An energized CO2 molecule could also re-emit a similar photon in the downward direction, and this new photon is re-absorbed by the earth, although this is far less likely. Photons absorbed and then re-emitted in an upward direction would not be distinguishable from those not absorbed in the first place.
When I made the statement that conservation of energy must hold for each frequency independently of the others, I was merely trying to provide the physical basis for applying Beer's Law to individual frequencies. But since you are convinced of that already, you can disregard this statement. This means that the energy flux contained in each frequency is proportional to the amplitude squared of the electric (or magnetic) field at that frequency, and the energy flux of the entire spectrum is simply the sum of the energy fluxes at the individual frequencies. This, of course, should be nothing new to persons knowledgeable in radiometry.
Now that we know that Beer's Law applies to the 15 micron absorption band, we see that this band is attenuated to insignificant values well below the TOA, with the electromagnetic energy being converted into thermal energy mostly within the atmosphere. Furthermore, adding more CO2 won't cause more warming because this band is already completely absorbed and converted into heat.
At this point, it looks like you have a few things you could mull over yourself from your comfortable chair and popcorn.
There is something concerning Kirchoff's Law that we all may have forgotten about. Actually, Charlie_Brown brought it up at 756.
Kirchhoff’s Law is absorptance = emittance (at thermal equilibrium) (emphasis added)
At this point, it must be remembered that a system in the middle of absorbing photons is not in thermal equilibrium since more energy is being added. After absorption, the newly introduced energy is re-distributed throughout the system (the atmosphere in our case) and IR spectrum in order to re-establish equilibrium. Now, the system is never far from equilibrium, but laws that are strongly conditioned on equilibrium may well be compromised.
CallItAsItIs :-
Absolutely no need to apologize for an "almost" double posting. After all, it did provide opportunity for a tad of humor.
[ If you happen to double-post in future, then simply do an additional brief post asking the Moderator to make one deletion at his early convenience. ]
Also ~ while our friend Michael Sweet is skeptical about your bona fides as a genuine poster . . . that is ultimately a matter for an umpire's decision by Moderators, who may (or may not) expunge the posts of clumsier trolls or of those whose crackpot ideas are expressed in a repetitive tiresome manner.
Some crackpots simply do a "drive-by" series of posts on SkS and then disappear ~ others will later phoenix themselves. But we hope they will then revise & renovate their contrarian arguments into something of at least 25% merit. # Sadly, I have never encountered a phoenix who manages to show that all the mainstream scientists are wrong. Yet we live in hope that AGW will someday prove to be a mistaken concept, eh.
I am still eating my popcorn, CallItAsItIs , while I wait for you to introspect about Motivated Reasoning. And I suspect that some of the reason you are here in this thread, is that the strictly rational part of your intellect is quietly hoping that the powerful emotional/non-rational part of you will be brought into agreement with the climate expert physicists. ( Ain't human psychology interesting ! )
[PS] This thread is on the usual downward spiral. CallItAsItIs you are ducking and avoiding the germaine questions and still apparently refuse to read refutations or clarifications. It is time put up or shut up. Ramanathan and Coakley 1978 https://ramanathan.ucsd.edu/wp-content/uploads/sites/460/2017/10/pr15.pdf put a clear early picture of solar flux. Since you strongly disagree, please give us your equations for describing the energy flux through a layer in atmosphere. Then we can compare with observations and decide scientifically.
CallItAsItIs @ 781:
Finally, we get to see something from you that is a little closer to reality, but once again you wander into interpretations that are blatantly wrong. Let's start with part of your second paragraph:
First of all, emission of IR radiation by CO2 (or any other gas in the atmosphere) is omni-directional. Up. Down. Left. Right. North. South. Southwest. All directions are equally probable. As a result, IR radiation is diffuse in nature - emission heads out in the shape of a sphere. It is convenient to consider this spherical problem in the context of two directions - a hemisphere we'll call "up" and a hemisphere we'll call "down". This leads to a well-known (for those of us informed on atmospheric radiation transfer) analysis named the two-stream approximation.
The key result is that at any height in the atmosphere, an IR photon emitted by CO2 has an equal probability of being emitted upward or downward. And yes, this "new" IR photon is indistinguishable from any other IR photon of the same wavelength. As such, it is just as easily absorbed as photons originating from the surface. In fact, we can calculate the probability of that absorption by applying - wait for it - Beer's Law, using the height of emission as the starting point and following the path through the atmosphere. If we want to include the emission of radiation and work it in with Beer's Law to get a more complete equation, we can get the Schwarzschild’s equation.
Way back when, you argued that CO2 in the atmosphere above 10m could not possibly absorb 15um photons because there weren't any left is now shown (by your own admission) to be completely wrong. There is a ready supply of 15um photons available, travelling in all directions. (You still vastly underestimate how many there are, and have missed out on the ones that are heading upward, but it's a start.) Earlier, you dismissed those upward-directed 15um photons as "thermal radiation" not related to CO2 - hopefully now you realize why we've been telling you that you are wrong. [But your paragraph 4 reduces that hope. See below.]
On to paragraph 3, which starts:
Frankly, that is bull$#!^. Conservation of energy has nothing to do with applying Beer's Law to individual frequencies. And no, we will not disregard that statement because it is yet another example of where you clearly do not understand the physics involved. Invoking an incorrect, imaginary explanation only serves to destroy your credibility.
Paragraph 4:
...and you are back to completely ignoring the observed fact that 15um radiation does exist in the upper atmosphere (moving in both upward and downward directions) in highly-significant values, and that CO2 can absorb these 15um photons (since you admit that these 15um photons are indistinguishable from ones emitted at the surface). And when you do the full math using the proper equations for absorption, emission, and conservation of energy, you find that adding CO2 does cause more warming.
Through the last 50-odd comments it is evident that the commenter CallItAsItIs is unable to accept that poly-atomic molecules in a gas will pick up vibrations from collision, this often enough that some of them will relax and emit a photon before further molecular collision. He seems to still believe that ideal gases exist. SO that's quite a pile of learning he would need to grasp to understand the greenhouse effect in terms of the molecular processes.
CallitAsItIs @ 782:
You say (your emphasis):
Actually, its thermodynamic equilibrium.
You go on to say (emphasis mine):
Translation: CallItAsItIs feels free to invoke Kirchoff's Law when he wants to, and ignore it when it is inconvenient to his bogus argument.
Let's look at a "radiometry textbook", since CallItAsItIs is so fond of them. I'll go back to Liu (1980) "An Introduction to Atmospheric Radiation", which I mentioned earlier as one of the books on my bookshelf. On page 13:
If CallItAsItIs wants to continue to ignore Kirchoff's law when forming his arguments, he will need to provide a much stronger argument as to why it does not apply than to hand-wave it away with a statement such as "may well be compromised".
A key point that explains why the CO2 band is not saturated has been lost in trying to convey fundamental concepts. To avoid semantics, I use the term “saturated” to mean no further effect from increasing CO2. This occurs when emittance = maximum value of 1.0. It should have been evident in Figure 3 of the link to my guest post that Bob provided earlier. It also should have been apparent when I said that the 15-micron peak at 50 km was below the Planck distribution temperature. The emittance of the strongest lines between 14.93-15.0 microns absorptance/emittance lines reach a value of 1.0 in the lower atmosphere and at the bottom of the stratosphere. However, the CO2 band has thousands of strong and weak emittance lines between 13-17 microns. The emittance of the 14.25-micron line has a value of 0.25 in the tropopause where it matters to energy loss to space. By Beer’s Law, the line will strengthen with increasing CO2. The 14.93-15.0 micron band may be saturated with respect to energy loss to space. However, the full range of the CO2 band is not saturated. The logarithmic band saturation effect is plotted in Figure 3 of my article in Chemical Engineering Progress that was referenced in the guest post.
CallItAsItIs, as a professor for an in-person class, I do not respond well when a student stands up and shouts “Wrong!” (@760), disrupts the class, and then repeats his misunderstanding. In person, that student would be dismissed. Let me know, with some respect, if you have any further questions.
Charlie_Brown @ 787:
CallItAsItIs has not shown much proclivity for following links and reading material people have pointed him to. (He doesn't even seem to read much of what people say in comments to this thread.)
Anyway, more to the point, since figure 3 in your guest post is a figure that is "available on the internet", you can easily re-use that figure and insert it into a comment, just like any other figure that is "available on the internet". I did this earlier for the Trenberth diagram, which is used on another SkS page.
The link to your figure is:
https://skepticalscience.com/pics/AtmosphericRadiationModel-Fig3-1200px.jpg
...and I can insert it here using the little "tree" icon on the Insert tab of the editor:
(In this case, I used the Appearance tab on the "insert Image"dialog box so that I could limit the display width to 500 pixels. This avoids breaking the web page formatting with large pictures.)
Bob Loblaw @772
When I made the statement that
...we are trying to determine the warming of the atmosphere due to GHGs tapping energy from the terrestrial IR radiation rising from the surface. This means that the upwelling terrestrial IR radiation is the source.
the term "upwelling terrestrial IR radiation" means all upward-bound IR flows shown in your diagram. Let's keep it straight as to what problem I am working and what problems I am not. And balanced terrestrial energy flows is one that I am not working on. Therefore, your chart is irrelevant. The problem I am working on is in determining how much of the 15 micron absorption band of CO2 is extinguished on its way from the surface to the TOA. And from what I have found, that figure is darn close to 100% regardless of the numbers on your diagram.
In regard to your statement that I ignore anything other than IR radiation, I can only say that I am studying the saturation of the 15 micron absorption band. Therefore, absorption at visible and UV radiation is irrelevant.
And since I was told to not forget about the sun as a source, I have an interesting question. It turns out that the same physics whereby CO2 blocks the 15 micron radiation from leaving the earth also blocks solar radiation at both the 15 micron and 4.3 micron bands from entering the atmosphere, which of course would cause cooling. Since these bands are of about equal spectral strength and since the solar irradiance is much stronger at 4.3 microns than the terrestrial, I wonder which one "wins".
Bob Loblaw @784
Frankly, that is bull$#!^. Conservation of energy has nothing to do with applying Beer's Law to individual frequencies.
All I can say to this is that all through my education and career, I have applied conservation of energy principles to individual frequencies and have seen many others do likewise in textbooks and papers, and it hasn't gotten me in trouble yet. Authors assume that readers already know this is perfectly valid. Mathematically, it works for linear problems, and Beer's Law is a linear equation. But if there is some other reason I am supposed to believe that it is legitimate for me to apply Beer's Law to different frequencies individually, then let's see it! Please note that I will view an unprofessional response involving sarcasm and/or ridicule as indicating that you are not up to the challenge!
...and you are back to completely ignoring the observed fact that 15um radiation does exist in the upper atmosphere (moving in both upward and downward directions) in highly-significant values,
I never said those 15 micron photons didn't exist. Now Beer's Law does predict vanishingly small intensities of this band in the upper atmosphere, but that is not all that is happening. Remember that Beer's Law is obtained by adding an absorption term to Maxwell's Equation which assumes a wave character of EMR. The upper atmospheric 15 micron photons are manifestations of the quantum nature of EMR. Now, I would be glad to further discuss this with you if you can respond in a non-insulting professional manner.
PS @783
CallItAsItIs you are ducking and avoiding the germaine questions and still apparently refuse to read refutations or clarifications.
What do you mean "still apparently refuse to read refutations or clarifications"? I have checked out every link and diagram that was posted, and only found two that were even remotely related to the problem I am addressing, and even those did not change my stand any concerning CO2 band saturation. The rest were either old, irrelevant, or just plain nonsense. For example, Eclectic's spiel about "Motivated Reasoning" or trying to psychoanalyze me can be skipped. In general, the other participants in this have been quite unprofessional in their communications regarding my postings. They have been sarcastic and insulting. They have accused me of self-contradictory assertions when it was they themselves who misunderstood me. They have stone-walled my arguments by claiming I didn't justify certain mathematical steps that have long been standard procedure in radiometry (See 784). Additionally, they accuse me of being "wrong" by bringing up material that is not relevant to the issue I am resolving, and then claiming I failed to include such material (see 772).
It gets quite interesting when I show them that their arguments against C02 band saturation violate the laws of thermodynamics, and I get no material response. Just so that you are aware, I am saving screen shots of this webpage from my first posting onward, and they may be used in a lecture series I am pulling together called Fear No Carbon. These lectures would explain this AGW non-science at both the scientist and layperson levels. So far, SkS has been my best source!
Now, I am still willing to correspond with the other participants on this webpage if they can do so in a professional manner. This means no insults or sarcasm, no psychoanalysis, and no accusations of evasion when I decline to respond to questions and inputs that are irrelevant or not applicable to my specific research topic. In the last paragraph of comment 787, Charlie_Brown asked me to show respect if I have further questions, and I intend to comply with his request. But I expect the same from others on this webpage. Otherwise, we have nothing further to discuss.
[PS] No, you manage to ignore the substance. Your arguments make no sense to me because you appear to ignore applying relevant bits of physics that contradict your view, and that applies in spades to other commentators. There is only way out of this - make predictions from your understanding of theory and compare with observations. I repeat - state your equation for energy flux through a layer of the atmosphere. Further discussison of photons are moot until you do so. As I understand your ideas, you would have considerable difficulties explaining observations like this: https://www.nature.com/articles/nature14240
CallItAsItIs @789,
There are three CO2 absorption/emission bands for IR (although it can get more complicated with massive rising CO2). At the temeratures found ion Earth, the 2.7 micron and 4.3 micron bands is too energetic to be anything more than an absorption band. And the 4.3 micron band is so weak from the sun that it is ignorable while the 2.7 micron band is strong enough to have a measurable dip in the incoming solar IR, but it is tiny.
More of a cooling influence from increasing CO2 is the central 15 micron wavelengths as these are not emitted into space until up in the stratosphere where temperature rises with altitude. But such central-15 micron cooling is far outweighed by the edges of the band's warming.
I noticed a chart from the science blogger Sabine Hossenfelder which you may find useful in describing the greenhouse effect (something which is not usually done well). The one word I would change is to substitute "impeded" for "trapped" in the 'grand description' line.
In addition to MA Rodgers's comment above :-
CallItAsItIs @789 :
Sorry for the Home Truth . . . but your comments are becoming more bizarre ~ you are implying that solar radiation penetrating to the lower atmosphere (see Trenberth's diagram with solar EMR being absorbed by dust, etc ) is somehow not warming the air at these lower altitudes. And in addition, you are implying that the CO2-related IR emitted/absorbed at the 0 - 10 meter altitude is incapable of warming the remainder of the atmosphere by means of kinetic motion and/or re-radiation.
and @790 :
You can apply "conservation of energy principles to individual frequencies" [unquote] and also you can apply COE principles to bands of frequencies . . . and indeed to all sorts of individual "trees" ~ but to get valid and useful results, you need to apply COE principles to the "forest" (i.e. the total atmosphere). If you do not do that total assessment, then you will fail to understand terrestrial climate.
and @791 :
Yes : "self-contradictory assertions" by the score.
If you blame readers "who misunderstood me" , then the fault is either your poor explanation of your New Physics of Climate . . . or that your New Physics is simply wrong.
( Though perhaps the Nobel Prize Committee will one day recognize & acclaim a third possibility,eh ! )
Oh, my. CallitAsitIs is doubling (tripling? quadrupling?) down on his misunderstandings of physics.
@ 789:
Now there's your problem. Balanced terrestrial energy flows are completely relevant to the greenhouse effect, the role of CO2 warming, global climate, etc.
Continuing in comment 790, in response to my pointing out that he continues to ignore 15um radiation in the upper atmosphere:
Let's see. In your very first post, On Nov. 24 (#722) (emphasis added):
Your second post, the same day (#723):
Your third post (Nov 25, # 726):
Your fourth post (Nov 25, #730) says it three times:
Your fifth post (Nov 25, # 740):
You then managed to make a few comments without repeating your error, but then it returns on Nov 26 in comment 751:
...and then on Nov 27, in comment 765:
On Nov 28:
Finally, on Nov 29, CallItAsItIs took a day off, but on Nov 30, he is back saying:
So, your claim that you "never said that those 15um photons didn't exist", is refuted by your daily claims that it either didn't exist ("there is no more", "have all been depleted"), or is insignificant/negligible/minuscule.
I particularly like the last part of that last statement I quoted: "...regardless of the numbers on your diagram." CallItAsItIs thinks that his fantasy fizziks trumps observations.
In short, CallItAsItIs dismisses huge amounts of relevant, critical, significant, and important theory and observations related to radiation transfer, global energy balances, and CO2-induced greenhouse warming by a wave of his hands, calling it "irrelevant".
If the facts disagree with CallItAsItIs's "theory", they must be disposed of.
I'll also respond to this bit from CallItAsItIs:
You mean a non-insulting, professional manner such as:
Frankly, your attitude since you got here has been condescending, confrontational, and tiresome. You have severe delusions of adequacy. Not only are there huge gaps in your knowledge, but much of what you think you know is just plain wrong.
Many of your blustering tactics may work in a group setting where people do not know the science, but here there are a few of us that do. You can find out more about me by looking at the "Team" menu item under "About" in the main masthead, but you are arguing with someone that has been studying climate for 45 years, took radiation transfer theory as a grad student 40 years ago, used to teach undergrad and grad climate courses as a professor, and spent a dozen years observing radiation at a climate research station.
The idea that you have something to teach me about radiation is laughable.
Bob Loblaw @784 and 795
Wonderful! Now with your radiation expertise and Schwarzschild's equation, you surely see that the solution for spectral intensity has a term that accounts for thermal radiation (ie. blackbody) and an exponential term that vanishes at high altitudes, giving us the exact same result I have been claiming through all the ridicule. Yes, those photons are there but they are there to establish thermal equilibrium at the surrounding temperature and not for warming. I'm glad you finally see the light!.
CallItAsItIs @ 796:
Wonderful. Now "establishing thermal equilibrium" has nothing to do with "warming".
Yes, I see the light. You simply cannot conceive of the idea that adding CO2 changes the temperature at which the atmosphere reaches "thermal equilibrium".
Do the actual math. Your handwaving achieves nothing other than making you look like a fool.
CallItAsItIs ~ may I humbly suggest that you present your New Physics at the WUWT website ["WattsUpWithThat"] .
At WUWT you would find a gratifyingly-large number of readers (and commenters) who will welcome your novel insights into the nature of Space-Time & the Universe in toto.
True, there will be some carping criticisms from WUWT-ites at the more educated end of the spectrum there. But on the whole, you will receive a very warm welcome from the majority of the spectrum ~ they are ever-ready to applaud anything which could seem to give a poke-in-the-eye to boring conventional mainstream science.
# But a warning, CallItAsItIs. Be quick to lap up the praise there . . . because, very soon, the loons crackpots and wingnuts at WUWT will wish to move on to the next pseudo-science-du-jour that promises to soothe the typical WUWT ego.
[ Loons crackpots and wingnuts . . . have I omitted anyone of the WUWT regulars ? . . . Wait, yes, there's a handful of actual scientific thinkers in the comments columns there, who post to enjoy tweaking the collective nose of the WUWT-ites . ]
CallItAsItIs has three fundamental blind spots that he does not understand, despite our addressing them several times. 1) He keeps referring to a single 15-micron band. Actually, as shown @788, there are thousands of absorptance/emittance lines for CO2 in the spectrum. Some are weak and some are strong, and Beer’s Law applies to each of them individually. 2) Once absorbed, near the surface or anywhere in the atmosphere, Kirchhoff’s Law applies, absorptance = emittance, and an equal number of photons are absorbed and emitted, although they are not the same identical photons. They do have the same intensity and wavelength. 3) Therefore, energy loss to space is determined by the uppermost radiating layer that “sees” space. The maximum value of emittance is 1.0. If a line reaches a value of 1.0, there will be more molecules above that altitude. If a line is less than 1.0 at the top of the troposphere, there will be more molecules at a lower altitude that is thicker and warmer. If the altitude reaches 0 km before the emittance reaches 1.0, then the remaining emitted energy will come from the surface.
There is more to CallItAsItIs’ misunderstandings, e.g., “Beer's Law is a linear equation.” No, it is exponential. And “their arguments against C02 band saturation violate the laws of thermodynamics.” No, they do not. But let’s get those first three blind spots resolved first. Continued repetition of misunderstandings without taking the time to study our explanations is not a sign of respect.
CallItAsItIs needs to do some self-study before he posts again, and certainly before he tries to teach this stuff. MA Roger @792 provides Dr. Sabine Hossennfelder’s summary Figure. Sabine also has a great entertaining video. My only quibble with her material is that she does not emphasize the strong and weak absorptance/emittance lines but refers to an average or effective altitude for all lines. I recommend again that CallItAsItIs studies the spectrum using the link that has already been provided twice for instruction and guidance. He needs to resolve the changing spectra with the step-by-step exercises. If he cannot resolve the results with his thinking, then he needs to think again before making more repetitive posting. Meanwhile, I have submitted another guest post that is in the review process. It describes the mechanism of warming that is similar to Sabine’s material, but it emphasizes the absorptance/emittance lines with upsetting and restoring the overall global energy balance.
Bob Loblaw @797
Yes, I see the light. You simply cannot conceive of the idea that adding CO2 changes the temperature at which the atmosphere reaches "thermal equilibrium".
Wrong! Whether adding CO2 changes the equilibrium temperature remains to be seen. I should note, however, that with the absorption strength of CO2 on the 15 micron band, one could probably show that band saturation occurs over a pretty wide temperature range.
Do the actual math. Your handwaving achieves nothing other than making you look like a fool.
I have already done the math but am not posting it here. Typesetting equations tends to be a long, grueling task for me and not worth the effort in view of the fact that you and your AGW comrades would most likely discredit it over statements I did not make or that you misunderstood. Attend my Fear no Carbon lectures if you want to learn something more about the mathematics of this band saturation effect.