What is methane's contribution to global warming?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
| |||
|
Methane plays a minor role in global warming but could get much worse if permafrost starts to melt. |
|||||
Climate Myth...
It's methane
"A United Nations report has identified the world's rapidly growing herds of cattle as the greatest threat to the climate, forests and wildlife. ...
...Livestock are responsible for 18 per cent of the greenhouse gases that cause global warming, more than cars, planes and all other forms of transport put together.
Burning fuel to produce fertiliser to grow feed, to produce meat and to transport it - and clearing vegetation for grazing - produces 9 per cent of all emissions of carbon dioxide, the most common greenhouse gas. And their wind and manure emit more than one third of emissions of another, methane, which warms the world 20 times faster than carbon dioxide." (Geoffrey Lean)
At a glance
Just like CO2, methane is a colourless, odourless gas. But the similarity ends there. Methane is highly reactive, to the extent that it can form a highly explosive mixture with oxygen. Methane explosions are a leading cause of mining disasters. For domestic use, the gas has an odour-producer added to it, so you can 'smell gas', in the event of a leak.
That reactivity is a good thing, since methane is a potent greenhouse gas, many times more effective at trapping heat in the atmosphere than CO2. It has caused almost a third of recent global warming. But methane oxidises quickly. The atmospheric lifetime of a molecule of methane is typically no more than 12 years. This is much shorter than the long atmospheric lifetime of CO2.
Due to that reactivity, the concentration of methane in the atmosphere is much smaller than that of CO2. For that reason, it is expressed in parts per billion (ppb). A thousand parts per billion is one part per million (ppm). Currently, the average methane concentration is 1894 ppb or 1.894 ppm. That is about 2.5 times pre-industrial levels.
Current sources of methane are a mixture of natural and manmade processes, according to the International Energy Agency. Man-made sources make up more than two thirds of the total. The key natural source is wetlands. However, there is also the poorly-understood potential for releases from methane hydrate deposits.
Methane hydrate, or methane clathrate as it's sometimes called, is a white, snow-like solid. Although it looks like snow, the resemblance ends there, because you can set fire to it. Methane hydrate occurs in marine sediments, where it forms during the bacterial decomposition of organic matter. Vast stores of the substance can build up in the sediment, over time.
Importantly though, methane hydrate is only stable at very high pressures and low temperatures. Such environments are typically found within the slopes that lead down from the continental shelves into the oceanic depths. Here, the water is deep and cold enough and there is still plenty of organic matter too. For methane hydrate, the conditions are perfect. Destabilisation of this buried 'flammable snow' could lead to methane release on a substantial scale. But it's important to bear in mind that this remains an incompletely-understood area - despite occasional scary headlines in the media. Vast-scale methane-release is regarded as very unlikely to occur under any plausible near-term emissions pathway.
Methane outgassing from melted permafrost is much better understood. You may have seen videos of methane ignition at lakes in permafrost-country. But currently, compared to man-made sources, this is still insignificant in the great scheme of things.
The leading source of man-made methane emissions is agriculture but the energy sector comes a close second. Waste treatment, in particular landfill, is also significant. Improvements are possible in all such sectors and are in some cases being implemented. Meanwhile, emitting CO2 at the rate of over 40 billion tons per annum, as we are now doing, still remains a seriously bad Idea. Methane should not distract us from that.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
While methane is a more potent greenhouse gas than CO2, there is over 220 times more CO2 than methane in the atmosphere - as of 2022, 417 ppm as opposed to 1.894 ppm. The amount of warming attributed to methane is calculated to be around 30% of the warming CO2 contributes. And the atmospheric concentration of both continues to rise (fig. 1).

Fig. 1: The continued rise in CO2 and methane. Global column-averaged CO2 and CH4 concentrations as measured by satellites, denoted XCH4, for 2003–2022 Monthly averages (red) and 12-months average (black). Redrawn from an original illustration at Copernicus, the European climate change agency.
Methane levels have increased more quickly than CO2 from the pre-industrial baseline concentration of some 700 ppb. That represents a 2.7 times increase, whereas CO2 has 'only' gone up by 50%. Man-made methane sources outnumber natural ones by about two thirds of the total. If we look at a breakdown of these, the key one is agriculture - in particular ruminant farming and rice-paddies. Although the exact figures vary according to the source of the information, a good ballpark figure is that 36% of anthropogenic methane emissions are due to livestock farming and rice cultivation alone. Coming close behind is the energy sector with 33% of emissions. Landfills and other waste treatment processes come third (fig. 2).
Progress is being made - in some sectors and in some countries - to reduce such emissions, but there is still a long way to go. As indeed pointed out by the myth-provider at the top of this page, some countries have better agricultural standards than others - and most of us understand that replacing rain-forests with cattle-ranches is about as insane as it gets. We should simply know better.
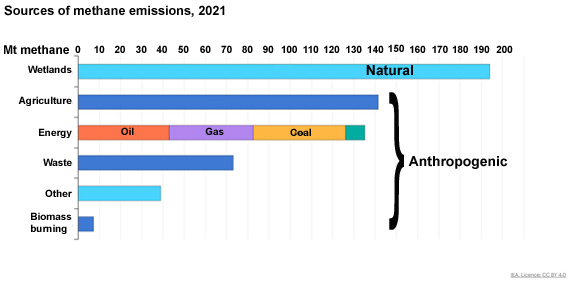
Fig. 2: Natural and anthropogenic sources of methane, in millions of tonnes. Figure redrawn from an original at the International Energy Agency website.
What about other methane sources that have featured in the news at times?
When permafrost thaws out, natural processes that were paused when it froze up are restarted, releasing both methane and CO2. As things stand, more work is required to quantify such methane sources, although their effects are well known and have been discussed many times here at Skeptical Science - just try entering 'permafrost' in the top left search bar to see!
There is also methane hydrate, or clathrate, to consider. Methane hydrate is a white, snow-like solid, composed of methane molecules trapped in cage-like structures formed from water molecules. The methane is generated by bacterial decomposition of organic matter such as the remains of plankton. Vast stores of the substance can build up in deeply buried marine sediments over time.
Methane hydrate is only stable at high pressures of 35 bars or more and at low temperatures, both confined to the world's deeper marine basins. What can destabilise methane hydrate deposits? Two things stand out: falls in overhead pressure and/or increases in local temperature. Pressure-falls can be brought about by a fall in sea level or by tectonic uplift of the sea-bed, both making the overhead water-column shallower. Temperature-increases can occur either through direct warming or changes in ocean circulation, or both. Any such change of circumstances that brings a methane hydrate deposit out of its stability zone could trigger its destabilisation, leading to significant outgassing of the methane.
These hazards remain to be fully understood but a lot of effort is going into investigating methane hydrate deposits and their potential role in sudden global warming, both in the past and potentially in future.
Methane should not be underestimated. Once in the atmosphere it has various effects and associated feedbacks that contribute indirectly to warming. Realclimate has an authoritative post detailing some of those, here.
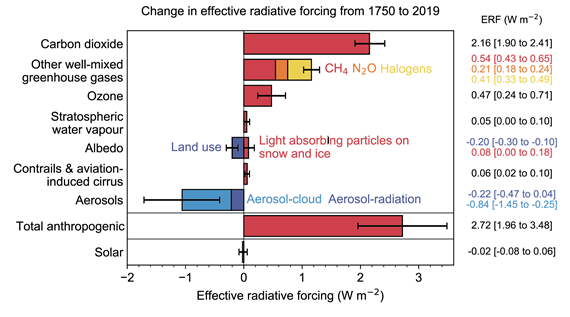
In AR6, the changes in radiative forcing due to methane and other greenhouse gases are presented (fig. 3). The figure shows that while CO2 is the biggest of our problems, methane is still significant and efforts to reduce its emissions should nevertheless continue to be implemented. But never at the same time let it distract from CO2. It's not a case of one or the other. They are both big problems requiring different solutions.
Fig. 3: Radiative forcing changes due to various agents, from 1750-2019. Graphic 7_6 from IPCC AR6 WGI Chapter 7
Last updated on 17 December 2023 by John Mason. View Archives































 Arguments
Arguments



































Does anyone know where I can find atmospheric CH4 levels on million-year timescales? For CO2 these are readily avilable.
Please note: a new basic version of this rebuttal was published on December 17, 2023 which includes an "at a glance“ section at the top. To learn more about these updates and how you can help with evaluating their effectiveness, please check out the accompanying blog post @ https://sks.to/at-a-glance