Is the CO2 effect saturated?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
The notion that the CO2 effect is 'saturated' is based on a misunderstanding of how the greenhouse effect works. |
|||||||
Climate Myth...
CO2 effect is saturated
"Each unit of CO2 you put into the atmosphere has less and less of a warming impact. Once the atmosphere reaches a saturation point, additional input of CO2 will not really have any major impact. It's like putting insulation in your attic. They give a recommended amount and after that you can stack the insulation up to the roof and it's going to have no impact." (Marc Morano, as quoted by Steve Eliot)
At-a-Glance
This myth relies on the use (or in fact misuse) of a particular word – 'saturated'. When someone comes in from a prolonged downpour, they may well exclaim that they are saturated. They cannot imagine being any wetter. That's casual usage, though.
In science, 'saturated' is a strictly-defined term. For example, in a saturated salt solution, no more salt will dissolve, period. But what's that got to do with heat transfer in Earth's atmosphere? Let's take a look.
Heat-trapping by CO2 in the atmosphere happens because it has the ability to absorb and pass on infra-red radiation – it is a 'greenhouse gas'. Infra-red is just one part of the electromagnetic spectrum, divided by physicists into a series of bands. From the low-frequency end of the spectrum upwards, the bands are as follows: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Gamma rays thus have a very high-frequency. They are the highest-energy form of radiation.
As our understanding of the electromagnetic spectrum developed, it was realised that the radiation consists of particles called 'photons', travelling in waves. The term was coined in 1926 by the celebrated physicist Gilbert Lewis (1875-1946). A photon's energy is related to its wavelength. The shorter the wavelength, the higher the energy, so that the very high-energy gamma-rays have the shortest wavelength of the lot.
Sunshine consists mostly of ultraviolet, visible light and infra-red photons. Objects warmed by the sun then re-emit energy photons at infra-red wavelengths. Like other greenhouse gases, CO2 has the ability to absorb infra-red photons. But CO2 is unlike a mop, which has to be wrung out regularly in order for it to continue working. CO2 molecules do not get filled up with infra-red photons. Not only do they emit their own infra-red photons, but also they are constantly colliding with neighbouring molecules in the air. The constant collisions are important. Every time they happen, energy is shared out between the colliding molecules.
Through those emissions and collisions, CO2 molecules constantly warm their surroundings. This goes on all the time and at all levels in the atmosphere. You cannot say, “CO2 is saturated because the surface-emitted IR is rapidly absorbed”, because you need to take into account the whole atmosphere and its constant, ongoing energy-exchange processes. That means taking into account all absorption, all re-emission, all collisions, all heating and cooling and all eventual loss to space, at all levels.
If the amount of radiation lost to space is equal to the amount coming in from the Sun, Earth is said to be in energy balance. But if the strength of the greenhouse effect is increased, the amount of energy escaping falls behind the amount that is incoming. Earth is then said to be in an energy imbalance and the climate heats up. Double the CO2 concentration and you get a few degrees of warming: double it again and you get a few more and on and on it goes. There is no room for complacency here. By the time just one doubling has occurred, the planet would already be unrecognisable. The insulation analogy in the myth is misleading because it over-simplifies what happens in the atmosphere.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
This myth relies on the use of a word – saturated. When we think of saturated in everyday use, the term 'soggy' comes to mind. This is a good example of a word that has one meaning in common parlance but another very specific one when thinking about atmospheric physics. Other such words come to mind too. Absorb and emit are two good examples relevant to this topic and we’ll discuss how they relate to atmospheric processes below.
First things first. The effect of CO2 in the atmosphere is due to its influence on the transport of 'electromagnetic radiation' (EMR). EMR is energy that is moving as x-rays, ultraviolet (UV) light, visible light, infrared (IR) radiation and so on (fig. 1). Radiation is unusual in the sense that it contains energy but it is also always moving, at the speed of light, so it is also a form of transport. Radiation is also unusual in that it has properties of particles but also travels with the properties of waves, so we talk about its wavelength.
The particles making up radiation are known as photons. Each photon contains a specific amount of energy, and that is related to its wavelength. High energy photons have short wavelengths, and low energy photons have longer wavelengths. In climate, we are interested in two main radiation categories - firstly the visible light plus UV and minor IR that together make up sunshine, and secondly the IR from the earth-atmosphere system.
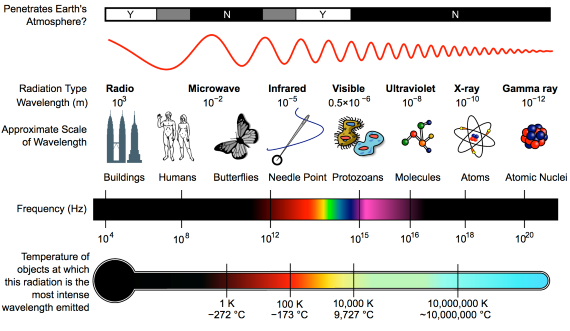
Fig. 1: diagram showing the full electromagnetic spectrum and its properties of the different bands. Image: CC BY-SA 3.0 from Wikimedia.
CO2 has the ability to absorb IR photons – it is a 'greenhouse gas'.So what does “absorb” mean, when talking about radiation? We are all familiar with using a sponge to mop up a water spill. The sponge will only absorb so much and will not absorb any more unless it's wrung out. In everyday language it may be described, without measurements, as 'saturated'. In this household example, 'absorb' basically means 'soak up' and 'saturated' simply means 'full to capacity'. Scientific terms are, in contrast, strictly defined.
Now let's look at the atmosphere. The greenhouse effect works like this: energy arrives from the sun in the form of visible light and ultraviolet radiation. A proportion reaches and warms Earth's surface. Earth then emits the energy in the form of photons of IR radiation.
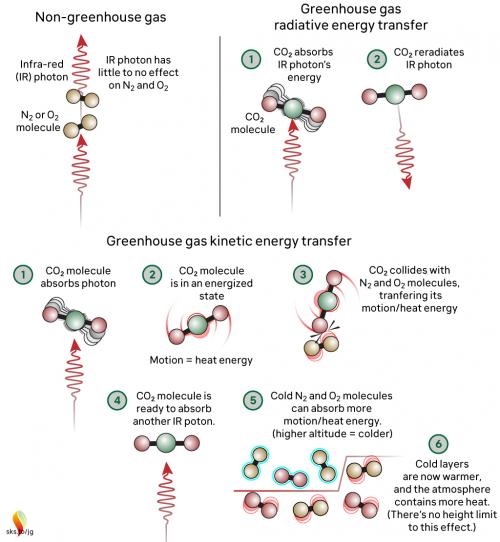
Greenhouse gases in the atmosphere, such as CO2 molecules, absorb some of this IR radiation, then re-emit it in all directions - including back to Earth's surface. The CO2 molecule does not fill up with IR photons, running out of space for any more. Instead, the CO2 molecule absorbs the energy from the IR photon and the photon ceases to be. The CO2 molecule now contains more energy, but that is transient since the molecule emits its own IR photons. Not only that: it's constantly colliding with other molecules such as N2 and O2 in the surrounding air. In those collisions, that excess energy is shared with them. This energy-sharing causes the nearby air to heat up (fig. 2).
Fig. 2: The greenhouse effect in action, showing the interactions between molecules. The interactions happen at all levels of the atmosphere and are constantly ongoing. Graphic: jg.
The capacity for CO2 to absorb photons is almost limitless. The CO2 molecule can also receive energy from collisions with other molecules, and it can lose energy by emitting IR radiation. When a photon is emitted, we’re not bringing a photon out of storage - we are bringing energy out of storage and turning it into a photon, travelling away at the speed of light. So CO2 is constantly absorbing IR radiation, constantly emitting IR radiation and constantly sharing energy with the surrounding air molecules. To understand the role of CO2, we need to consider all these forms of energy storage and transport.
So, where does 'saturation' get used in climate change contrarianism? The most common way they try to frame things is to claim that IR emitted from the surface, in the wavelengths where CO2 absorbs, is all absorbed fairly close to the surface. Therefore, the story continues, adding more CO2 can’t make any more difference. This is inaccurate through omission, because either innocently or deliberately, it ignores the rest of the picture, where energy is constantly being exchanged with other molecules by collisions and CO2 is constantly emitting IR radiation. This means that there is always IR radiation being emitted upwards by CO2 at all levels in the atmosphere. It might not have originated from the surface, but IR radiation is still present in the wavelengths that CO2 absorbs and emits. When emitted in the upper atmosphere, it can and will be lost to space.
When you include all the energy transfers related to the CO2 absorption of IR radiation – the transfer to other molecules, the emission, and both the upward and downward energy fluxes at all altitudes - then we find that adding CO2 to our current atmosphere acts to inhibit the transfer of radiative energy throughout that atmosphere and, ultimately, into space. This will lead to additional warming until the amount of energy being lost to space matches what is being received. This is precisely what is happening.
The myth reproduced at the top – incorrectly stating an analogy with roof insulation in that each unit has less of an effect - is misleading. Doubling CO2 from 280 ppm to 560 ppm will cause a few degrees of warming. Doubling again (560 to 1130 ppm) will cause a similar amount of additional warming, and so on. Many doublings later there may be a point where adding more CO2 has little effect, but recent work has cast serious doubt on that (He et al. 2023). But we are a long, long way from reaching that point and in any case we do not want to go anywhere near it! One doubling will be serious enough.
Finally, directly observing the specific, global radiative forcing caused by well-mixed greenhouse gases has - to date - proven elusive. This is because of irregular, uncalibrated or limited areal measurements. But very recently, results have been published regarding the deep reinterrogation of years of data (2003-2021) from the Atmospheric Infrared Sounder (AIRS) instrument on NASA's Aqua Satellite (Raghuraman et al. 2023). The work may well have finally cracked the long-standing issue of how to make finely detailed, consistent wavelength-specific measurements of outgoing long-wave radiation from Earth into space. As such, it has opened the way to direct monitoring of the radiative impact (i.e. forcing + feedback) of greenhouse gas concentration changes, thereby complimenting the Keeling Curve - the longstanding dataset of measured CO2 concentrations, down at the planet's surface.
Note: Several people in addition to John Mason were involved with updating this basic level rebuttal, namely Bob Loblaw, Ken Rice and John Garrett (jg).
Last updated on 31 December 2023 by John Mason. View Archives































 Arguments
Arguments





































Eclectic @442
I'm afraid bobhisey is correct in the statement that no 14-16 micron radiation leaves the earth — at least not from its surface. Due to the strong absorption of such radiation by CO2, the intensity of this band (radiating from the surface) is insignificant at the altitude of orbiting satellites. Therefore, any measurements of these wavelengths taken aboard satellites fall into the category of upper atmospheric and space physics. From what I have read, this radiation from space appears somewhat as a blackbody at temperature 220 deg. K. Anyway, it has nothing to do with the greenhouse effect at lower altitudes.
[BL} I'm afraid that you make the same basic error that bobhisey and many other people commenting on this thread have made. At least you add the "not from its surface" clause, but you completely ignore the well-known and observed fact that the lower atmosphere that is absorbing IR radiation in those wavelengths is also emitting it in those wavelengths.. as a result, measurements of upwelling IR radiation in those wavelengths always shows a continuous stream, from the surface up to the top of the atmosphere.
DragonHeads @700 and @701 ,
You are right that the bucket-of-water analogy is ugly & unlikable (IMO).
But you are wrong about the GreenHouse Effect [GHE]. Where do you get the idea that the GHE regarding CO2 is concerned only with the 15 micron IR emission from the surface or near surface [say, at the 2 meter height] ???
Bobhisey [and here I am hoping I am not treading on the toes of Present Company ] is basically & stubbornly clueless about GHE.
The GHE involves the whole planet ~ which includes the full depth of the atmosphere. Energy in & energy out, at the so-called Top Of Atmosphere [TOA]. Unfortunately, the TOA term is a tad misleading (just as is the GHE term) . . . but if you take the trouble to think all these climate concepts through, then it becomes "bleeding obvious" that the scientists are correct.
DragonHeads, please slow yourself down ~ and clarify where you think the atmospheric physicists are wrong. ( And perhaps it might be best to assure sensitive readers that no Sky Dragon Slayers have been harmed in your explanation. )
[BL] There are strong indications that Dragonheads is yet another sock puppet of a large collection of previously-banned users. As this is a violation of the Comments Policy, this user will not be participating any more.
Moderator: Bob, thanks ~ the indications were indeed strong.
While there is some entertainment value in replying to those delusional puppets . . . nevertheless I am quite happy for my own posts (including this) to be deleted from this thread. Maybe after a 24 or 48 hour delay, in order to allow a wider viewing of the shenanigans?
Eclectic and Moderators
If you would quit being so arrogant and ban-happy, you might just learn a little something on subject you are talking about. From your comments, analogies, and "rebuttals" on the CO2 greenhouse effect saturation issue, I would place your knowledge of spectroscopy somewhere between lacking and non-existent! Since all GHGs, including CO2, are trace gases, their spectra are much closer to that of single molecules than blackbodies. As the concentration of such a gas increases, however, the individual spectral lines are broadened into bands. If the density is further increased, those bands eventually overlap each other and are merged into a more or less continuous spectrum. Finally, in the extremely high density limit, the spectrum approaches a blackbody and it actually makes sense to talk about a temperature and pressure of the gas.
Now, the spectra of the main atmospheric gases N2 and O2 can be approximated with blackbody curves since they are of sufficiently high concentration. Also, the absorption coefficients can be regarded as roughly constant over the IR range. This is not the case, however, for CO2 nor any of the GHGs since these are trace gases. For the GHGs, we must determine an absorption coefficient for each spectral band. In the case of CO2, the important band for the GHE is the 2 micron band at a 15 micron wavelength. All other bands are either too weak or too far away from the peak of the upwelling IR spectrum. Therefore, CO2 can affect temperature only by absorbing radiation within this particular band. This is a strong absorption, however, so radiation within this band of the upwelling thermal energy is depleted at altitudes of only a few hundred meters. Above that, CO2 can absorb no more upwelling energy regardless of its concentration. Now, there is still upward-bound thermal energy, but not within the 15 micron band. That energy would most likely be absorbed by H2O vapor or escape to outer space.
Unfortunately, however, every purported rebuttal to CO2 GHE saturation in this Climate Myth page has, one way or another, involved the assumption of a single absorption coefficient for CO2 which applies for the entire IR spectrum. Simply put, this is incorrect and results in gross over-estimations of the amount of heat energy absorbed by CO2.
In summary, your case against CO2 GHE saturation wouldn't stand up under the scrutiny of a good student.
[PS] You can lead a horse to water...
Welcome back, DragonsHeads / DragonHeads / DragonSeed / DragonTeeth... It appears you have enlisted the aid of ChatGPT or Bard or other Artificial Intelligence "large language models", to construct your post #704.
ChatGPT etcetera typically produce a lot of words, with an initial semblance of meaning . . . but on closer examination, the words can often fail to show a true connection with reality ~ and that is the case here. DragonChat, you are spouting nonsense. Come back in 2030, in your seventh iteration ! [ Meanwhile, you might enjoy exercising yourself at the website WattsUpWithThat ;-) ]
Moderator ~ you are too quick on the draw !
Eclectic @705,
If the jibberish presented @704 is an output from ChatGPT, or some similar machine learning engine, then the technology is being greatly over-hyped.
I don't see that the CO2 absorption/emission spectrum "approaches a blackbody" spectrum at high concentration or density. (Concentration and density seem to be confused in the #704 account.) N2 & O2 are transparent to IR so they have no spectra (although this is indeed "constant over the IR range"). There are more bands of the spectrum absorbed by atmospheric CO2, not just at 15 micron. And up-welling IR is not restricted to "altitudes of only a few hundred meters" as CO2 emits as well as absorbs.
I've played around with ChatGPT and found that it has a tendency to make sh*t up. When you push further it will acknowledge that it is merely a natural language generator. It doesn't have the capacity to review and verify the accuracy of its output. It's just very good at sounding authoritative. But, to its credit, it will tell you not to rely on its statements and to engage experts in the field to verify any facts.
[ If the Moderator will allow a brief off-topic musing, I promise a sort of return in my concluding paragraph. ]
As a complete tyro in the world of probabilistic AI language generative models, I picture ChatGPT as the analogy of a Zillion monkeys tapping away at a Zillion typewriters . . . and eventually (which is actually a millisecond) out comes something speciously good. The product is sometimes Booker Prize standard; sometimes merely quite presentable; sometimes a diamond but deeply flawed when examined closely; and sometimes there is an Einsteinian Pearl of inventiveness (if the reader has the wit to pick it up and run with it). But always, the winning monkey has no real knowledge of what he has produced. Play-It-Again-Sam . . . and a millisecond later, the new winning monkey gives you a somewhat different product. ~In a decade's time, will the current language AI become so refined as to filter out its own fabrications & nonsenses? Probably yes...
On-topic ~ another analogy is the brain of the climate-science-denier, whose Motivated Reasoning (produced by a Zillion monkey neurons) keeps coming out with flawed presentations, in various repetitions. Monkeys, or Dragons?
Thanks, doesnt venus provide some evidence of what levels of CO2 would be needed to saturate?
chuck22 @709,
I would suggest it is more that Venus shows what a thick atmosphere does to climate while Mars shows it for a thin atmosphere. Both have an atmosphere comprising about 95% CO2. Yet the surface of Mars has zero GH-warming while on Venus it is an impressive +407ºC.
Venus has about 80% of the solar warning relative to Earth, this due to its higher albedo (left hand graphic below) which more than compensates for being closer to the Sun. Thus the "naked planet" temperature for Venus (230K) is lower that Earth's (254K). Venus has a 92 bar atmosphere and the clouds in such a thick atmosphere are a major insulation mechanism preventing IR across the entire spectrum from escaping to space from anywhere near the surface.
Zhong & Haig (2013) show (their Fig6b) that the climate forcing on Earth from CO2 (which at 389ppm provides with feedbacks GH-warming of +34ºC) would be perhaps trebled by CO2 levels up near the 90% mark, (Fig6b shows the direct forcing up to ~30% CO2) an unrealistically high level, but it does show that additional CO2 does not "saturate".
In recent interactions regarding the topic of saturation, I have on a few occasions now been directed to a relatively recent paper by Wijngaarden and Happer (Dependence of Earth's Thermal Radiation on Five Most Abundant Greenhouse Gases, arxiv.org/abs/2006.03098). Now, I know Happer at least has enough history to warrant extreme skepticism and that, if he really had any new insights, this work would probably have had more of an impact. On the other hand, I am not by any stretch of the imagination an expert and am unable to pinpoint what is wrong with what he is saying. Much of what is in the paper seems to be "standard" physics but at some point his conclusions diverge from what I understand from elsewhere. I wondered if anyone here could offer any insight.
Please accept my apologies regarding my previous post. It looked initially as if there had been no comments here since 2019. Once I had posted, I saw that there were some more recent pages of comments and I have just seen that this paper has been discussed.
[BL] Yes, the comments section on this thread is long and convoluted. Navigation is not as easy as we'd like.
Note that Skeptical Science also has an internal search function, available just under the masthead on the upper left of each page. You can use that to find material, and it will search the contents of comments if you follow the button on the results page (which at first only presents hits in our blog posts and rebuttals).
Please note: the basic version of this rebuttal has been updated on December 31, 2023 and now includes an "at a glance“ section at the top. To learn more about these updates and how you can help with evaluating their effectiveness, please check out the accompanying blog post @ https://sks.to/at-a-glance
Thanks for the heads up re Raghuraman et al. 2023 in the new "basic" section.
Having looked at CERES data in the past I now find myself wondering why (apparently) nobody has previously thought of using AIRS data to "provide measurements of Earth's emitted thermal heat at fine-scale wavelengths".
Am I missing something? If so, what?!
Jim: I have not had time to read that paper in detail yet, but I can provide a bit of background.
From the paper, it mentions
In order to get spectral data from a light source, the light source needs to be split into different wavelengths.
The raw diode array output needs to get translated to spectral irradiance, and the spectral resolution is limited to the number of individual diodes in the array.
The Raghuraman et al paper then increases the resolution using radiance models as an interpolation/enhancement method, and uses CERES broadband (not spectral) data to help limit the model results.
In short, it's not a particularly simple process. Not surprising that it has taken time for someone to do it.
JockO @ 711 This is a long and convoluted thread, but your question is a very good one. Recently I had another occasion to find the answer. Upon reviewing the many comments since 2019, the concept of saturation has been discussed thoroughly and does not need to be repeated. I also got involved with that between @669 and @679. However, the specific problem with Wijngaarden & Happer has not been pinpointed previously.
W&H describe the physics of radiant energy and the effect on the spectrum of outgoing infrared radiation very well, not withstanding a complex and distracting diversion into the atmospheric temperature profile. However, they make a misleading comparison to reach a false conclusion that “at current concentrations, the forcings from all greenhouse gases are saturated.” They compare the effect from 0-400 ppm with 0-800 ppm, both of which include the very steep initial slope of the band saturation effect, to conclude that the current rate of global warming is negligible. But the initial steep slope is irrelevant to anthropogenic global warming. In W&H Figure 4, they illustrate and compare the difference in the green line (0 ppm) and the black line (400 ppm) to the difference between the green line and the red line (800 ppm). To describe global warming, they should be comparing the difference between the black line and the red line. Thus, they use an irrelevant comparison to reach an incorrect conclusion “at current concentrations, the forcings from all greenhouse gases are saturated.” Saturated should mean no change as it would to a lay person, not diminishing change, although even the semantics of the definition are debated and misleading. In any case, anthropogenic global warming is not negligible.
I have a question concerning the Advanced Rebuttal for this "Is the CO2 effect saturated?" argument. I agree that thermal energy is spread around and transferred upward by radiation and convection and that IR emissions are occurring at all levels in the atmosphere. What is not mentioned, however, is where and how the CO2 molecules absorb IR energy from the 15 micron band for release as thermal energy in the greenhouse effect.
[snip] Could someone clarify this?
[BL] These and many other questions you have had over the years, in many sock puppets, have been clarified over, and over, and over again. Nothing will every make it into your closed mind, so why bother?
NavierStokes @717 :
Your question is answered in the Basic version of the Rebuttal.
Eclectic@718:
Whoever wrote the Basic Rebuttal doesn't understand the greenhouse effect at all. They seem to believe that the GHG molecules absorb IR radiation directly from the incoming sunlight instead of the upwelling terrestrial IR from the surface as indicated in the following quote:
Sunshine consists mostly of ultraviolet, visible light and infra-red photons. Objects warmed by the sun then re-emit energy photons at infra-red wavelengths. Like other greenhouse gases, CO2 has the ability to absorb infra-red photons.
Remember that 99%+ of the incoming EMR from the sun is in the visible spectrum and is absorbed by the earth (except of course for what is reflected as albedo). The earth then re-emits this absorbed energy as a 288-294 deg. K blackbody at the surface. We then get the greenhouse effect when the GHG molecules absorb this upward-bound IR and convert it into thermal energy in some manner. Therefore, this Basic Rebuttal badly needs to be rewritten and my question still stands.
[Snip]
[BL] The people writing the rebuttals here understand the greenhouse effect far better than you and your many sock puppets do. It is unfortunate that your stubbornness prevents you from ever learning any of the many things you clearly do not understand.
NavierStokes @719. :
The basic principles of absorption/emission and kinetic transfer of energy are (in the OP) set out and illustrated in a simple manner which allows the reader to understand the obvious implications.
So to that extent, your question is moot (= void).
If you wish to re-write the Basic Rebuttal in a superior form, please post it here as a demonstration. Readers would doubtless be interested to review your efforts ~ and the Editors may well accept a superior replacement, or at least make some modifications to the OP.
Per Ardua Ad Astra.
[BL] Unfortunately, NavierStokes is yet another sock puppet of a user that has polluted these threads over the years. As sock puppetry is a violation of the Comments Policy, NavierStokes will no longer be participating here - until he makes yet another attempt to break the rules and create another sock puppet (forcing us to ban him again...)
Moderator Bob , thank you for the notification of sock-puppetry.
Passing strange, is it not, that whenever a certain anonymous author (under numerous pseudonyms) keeps arguing that 20+20=37 . . . he keeps assuming that the editor won't recognise the foolish mathematical error being repeated year after year.
One is reminded of Einstein's definition of insanity.
I would like to make a few comments concerning the following paragraph in the Further details section.
Greenhouse gases in the atmosphere, such as CO2 molecules, absorb some of this IR radiation, then re-emit it in all directions - including back to Earth's surface. The CO2 molecule does not fill up with IR photons, running out of space for any more. Instead, the CO2 molecule absorbs the energy from the IR photon and the photon ceases to be. The CO2 molecule now contains more energy, but that is transient since the molecule emits its own IR photons. Not only that: it's constantly colliding with other molecules such as N2 and O2 in the surrounding air. In those collisions, that excess energy is shared with them. This energy-sharing causes the nearby air to heat up (fig. 2).
This is correct but it should also be noted that the absorption spectrum of CO2 is quite different than that for H20 vapor. In the case of CO2, strong absorption occurs but primarily in the (narrow) 15 micron band. Absorption or emission of IR by CO2 outside this band is generally considered to be small. For H20, however, we find weaker absorption but it is much more evenly spread out over the entire IR spectrum. Therefore, the CO2 greenhouse effect is determined primarily by what happens in the 15 micron band.
In the case of CO2 and the 15 micron absorption band, the N2 and O2 molecules in the surrounding air collide with energized CO2 molecules which causes the extra energy (from absorbed photons) to be converted into thermal energy, thereby raising the air temperature. Once this warming occurs, however, the upwelling 15 micron IR energy is reduced by the amount of thermal energy that was released in the collisions involving CO2 molecules. Otherwise, energy would not be conserved. Band saturation occurs if the upwelling 15 micron radiation is reduced to negligible values at some altitude below the TOA. Above this altitude, there is no more upward-bound IR energy that CO2 molecules can absorb. Essentially, the entire 15 micron band has been absorbed and additional CO2 would not cause further greenhouse warming.
I have a question about the video posted in the Intermediate rebuttal. At about 2:38 into this video, Mr. Richardson states "in the upper layers of the atmosphere, the greenhouse effect isn't saturated". I need clarification on this since band saturation is not an altitude dependent quantity. As I understand it, the greenhouse effect is saturated for a particular GHG if there is an altitude at which the absorption bands for that GHG have all been depleted (from the upwelling IR radiation) through absorption at lower altitudes. Therefore, a GHG is saturated for a particular atmospheric profile of the GHG, or it isn't. It's not saturated at one altitude but not another. Such statements don't make sense.
CallItAsitis @ 722 and 723:
Unfortunately, your understanding is incomplete and makes a common error by many that have tried to argue that the CO2 effect is saturated.
Your error is most easily seen in your statement "Above this altitude, there is no more upward-bound IR energy that CO2 molecules can absorb." This simply is incorrect, because your statements completely ignore the fact that CO2 also emits IR radiation. As long as CO2 is emitting IR radiation (at 15um and any other wavelength where CO2 is active), there will continue to be an upward-directed flux of IR radiation at those wavelengths. Since CO2 is present throughout the atmosphere, you will never, ever, see an altitude at which there is no upward flux of 15um radiation.
As long as you ignore the emission of IR radiation by CO2, you will fail to understand the greenhouse effect, and fool yourself with respect to "saturation". Your definition of "saturation" looks only at surface-emitted IR radiation and whether it can pass through the entire atmosphere in one go. It is your view of "saturation" that makes no sense.
As an FYI, the IR flux vs altitude question (both upwelling and downwelling) can be explored using the online MODTRAN model. To start, I suggest reading this blog post guest-authored by frequent reader/commenter Charlie Brown.