Ocean acidification: global warming's evil twin
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
| |||
|
Ocean acidification threatens entire marine food chains. |
|||||
Climate Myth...
Ocean acidification isn't serious
'Our harmless emissions of trifling quantities of carbon dioxide cannot possibly acidify the oceans. Paper after paper after learned paper in the peer-reviewed literature makes that quite plain. Idso cites some 150 scientific sources, nearly all of them providing hard evidence, by measurement and experiment, that there is no basis for imagining that we can acidify the oceans to any extent large enough to be measured even by the most sensitive instruments.' (Christopher Monckton)
At-a-glance
Have you heard of ocean acidification? Does it mean that if you go swimming in the sea, you are liable to dissolve? No. You'll be OK because you are not a calcifying organism, such as a mollusc, a coral or a sea-urchin.
So why is ocean acidification serious? Because it can potentially lead to massive collapse of marine food-chains. Let's take a look at what the term means.
The pH scale, which measures acidity and alkalinity of water-based chemical solutions, runs from 0 (highly acidic) to 14 (highly alkaline), with pH 7 being the neutral halfway point. Importantly, the scale is logarithmic, meaning that a jump of one point towards zero means a tenfold increase in acidity.
Acidification simply means lowering the pH value from any point on the pH scale towards zero. It's similar to the way we talk about temperatures. If the pH of a solution shifts from 9 to 8, that is acidification, even though the pH is still on the alkaline side of neutral. Likewise, if the temperature rises from -40°C to -15°C, it has noticeably warmed, even though it's still darned cold.
Now, typical seawater is slightly alkaline at around pH 8.1. Rainwater, which always contains dissolved carbon dioxide (the old name for which was 'carbonic acid gas'), has a more acidic pH of around 5.6. You have likely visited or watched footage of spectacular caves, have you not? All carved out by carbonic acid, dissolving solid limestone over many thousands of years.
Carbonic acid is not only present dissolved in raindrops. It also forms by the dissolving of carbon dioxide at the air-water interface of our oceans. The more carbon dioxide in the air, the more goes into the oceans, driving their pH from 8.1 downwards. Now, the huge problem this creates, well before we get anywhere near the neutral value, is as follows.
Many marine organisms build and maintain their protective shells or skeletons from 'biogenic' calcium carbonate. The word biogenic means made by living things. These creatures extract the calcium and carbonate ions dissolved in seawater and combine them together. Under normal conditions, such calcium carbonate is stable in shallow waters. That's because dissolved carbonate ions are present in such high concentrations that the waters are said to be saturated with them.
But if seawater pH falls, even by a small amount, the concentration of dissolved carbonate ions falls. When that happens, biogenic calcium carbonate becomes more soluble and can start to dissolve. Depletion in dissolved carbonate ions thus makes it harder for such organisms to maintain their protective or skeletal structures. In the worst case scenario, the rate of calcium carbonate dissolution is faster than its formation. When that happens, mass-mortality of calcifying organisms can occur.
We're talking about critters that underpin entire marine food-chains here. Things from near-microscopic calcifying plankton to shellfish, lobsters and crabs the seafood we eat in other words. That's why ocean acidification is deadly serious.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
Not all of the CO2 emitted by human industrial activities remains in the atmosphere. Between 25% and 50% of these emissions over the industrial period have been absorbed by the world’s oceans, preventing atmospheric CO2 buildup from being much, much worse. But this atmospheric benefit comes at a cost.
As ocean waters absorb CO2 they become more acidic. This does not mean the oceans will become like the acids one encounters in a chemistry lab. However, marine life can be highly sensitive to slight changes in pH levels and any drop in pH is an increase in acidity, even in an alkaline environment. Worse, the pH scale is logarithmic, meaning that for each single-digit decline in pH, acidity (defined as hydrogen ion activity) rises tenfold.
Surface seawater pH has been relatively stable over recent geological time, fluctuating between cold glacial periods (pH 8.3) and warmer interglacials (pH 8.2). But since the Industrial Revolution, average seawater pH has dropped towards a recent figure of less than 8.06, an approximately 30% increase in acidity (fig. 1). This is a faster change than any over the past 50 million years (Rhein et al, 2013, available from IPCC here).
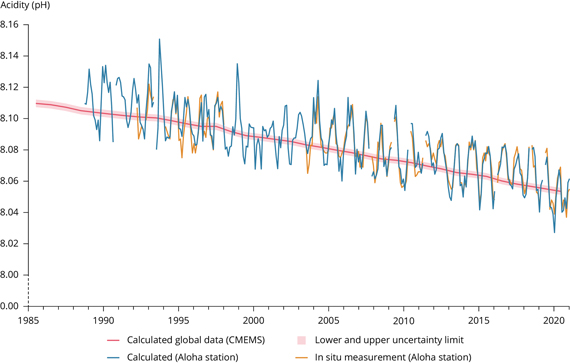
Fig. 1: Decline in ocean pH measured at the Aloha station (in the Pacific Ocean off Hawaii) and yearly mean surface seawater pH reported on a global scale Source: European Environment Agency (Copernicus Marine Service).
Because of its inextricable link with CO2 emissions, this rate of acidification is projected to accelerate even further through the 21st century under a business-as-usual scenario with potentially catastrophic impacts to marine ecosystems (Bindoff et al. 2019 (PDF from IPCC)). These trends are becoming clearer globally.
According to the IPCC's Sixth Assessment Report (AR6), there is, " a very likely rate of decrease in pH in the ocean surface layer of 0.016 to 0.020 per decade in the subtropics and 0.002 to 0.026 per decade in subpolar and polar zones since the 1980s. Ocean acidification has spread deeper in the ocean, surpassing 2000 m depth in the northern North Atlantic and in the Southern Ocean (fig. 2)."
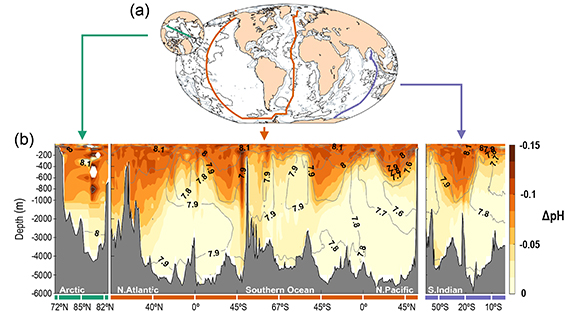
Fig. 2: Spread of ocean acidification from the surface into the depths since pre-industrial times. (a) Map showing the three transects used to create the cross sections shown in (b), showing the vertical sections of the changes in pH between 1800–2002 due to anthropogenic CO2 emissions; the darker the colours the greater the change. Contour lines are their contemporary values in 2002. Graphic sourced from IPCC AR6. (Lauvset et al. 2020).
Such changes in ocean chemistry, if allowed to occur, will be irreversible for many thousands of years. The biological consequences could last much longer.
How do we know that? Through the geological record. When mass-extinctions have occurred, most of them are tied-into unimaginably severe episodes of volcanism, at a scale never witnessed by humans. But the carbon footprint of such cataclysms has in fact been similar to our own. And what do we see as a consequence of such events? The fossil record shrinks in terms of its biodiversity and there are what we call 'reef-gaps', periods of several million years during which coral reefs - large highly diverse colonies of corals and myriad other species - were to all intents and purposes absent.
The reason why reef-gaps occur at such times is because as surface waters become more acidic, it becomes more difficult for corals, shellfish and other calcifying organisms to form and maintain the hard calcium carbonate skeletons or shells necessary for their survival. When things start getting really bad, that calcium carbonate dissolves away as fast as it can be deposited - that means curtains for such critters.

Fig. 3: just some of the life-forms at deadly risk from the acidification of near-surface ocean waters.
Coral reefs provide a home for more than 25% of all oceanic species, so you can see why this matters so much. Some calcifying organisms, such as the tiny pteropods (fig. 3), underpin many oceanic food chains: take them out of the system and down those food-chains come crashing. Many communities around the world, constituting millions of people, are at the apex of such food-chains, relying on seafood as part of a healthy diet. You should now be able to see the problem. Like a thief in the night, ocean acidification is creeping up on us, while we sleep on in blissful unawareness.
Last updated on 25 June 2023 by John Mason. View Archives































 Arguments
Arguments




































A question on ocean acidification from a non-scientist historian who's abidingly concerned about anthropogenic global climate disruption (and teaches freshman college students about this stuff in a first-year seminar on "People & the Planet" at a small liberal arts school in Central PA):
I'm reviewing climate change denialist Gregory Wrightstone's book, "Inconvenient Facts" (2017), and I'm a bit puzzled by one of his assertions. On p. 110 he writes:
"During the Cambrian, Ordovician and Silurian periods of the early Paleozoic era (543-416 million years ago), CO2 usually exceeded 4,000 ppm, reaching a maximum of nearly 8,000 ppm in the Cambrian period. The later was ~20 times today's concentration. When we compare CO2 levels to the rock record from the author's home turf in the Appalachian Basin of the eastern United States, we find that most of these CO2-enriched periods were dominated by limestone deposition. Limestone deposition could not have occurred had the oceans been 'acidified'. Most of the limestone was deposited during the periods of highest CO2 concentrations."
Thanks to this website and the references in this comments section, I've been able to find ample evidence discounting most all of Wrightstone's other assertions on ocean acidification, but this one has me puzzled. How were marine organisms able to make hard shells, and deposit massive amounts of limestone, when atmospheric CO2 (and oceanic carbonic acid levels) were so high? It's my understanding that when atmospheric CO2 reaches ~550 ppm, CO2 absorption by the oceans & the spike in oceanic carbonic acid levels renders marine animals incapable of forming hard shells. So how were these huge limestone deposits created when atmospheric CO2 levels (and oceanic carbonic acid levels) were so high?
Thanks in advance for helping me (and my students) understand the science involved here.
Michael Schroeder, I'll try to answer your question from the chemical point of view.
At first, please note that only a small part of dissolved CO2 exists in seawater as CO2(aq). According to contemporary experimental data, ~90% of dissolved inorganic carbon is in the form of HCO3-, ~10% CO2-3, and only ~0.5% CO2(aq).
The second. Only very small fraction of CO2(aq) (about 1/1000) converts to the carbonic acid H2CO3 , and eventually only a small part of H2CO3 dissociates to form hydrogen ions H+. Chemical calculations show that the effect of CO2 on seawater acidity accepted
by the majority of climatologists is highly exaggerated.
Conversion of absorbed carbon dioxide to bicarbonate and carbonate ions is determined by total alkalinity of seawater that in considered geological period was, perhaps, not less than now. Possibly in that time ratio carbonate/bicarbonate was greater, because at higher temperatures bicarbonate converts to carbonate:
2HCO3- → CO2-3(aq) + H2O + CO2
So, limestone deposition at high atmospheric concentrations of carbon dioxide is
quite understandable.
[Rob P] Conventional chemistry explains why the ancient oceans were hospitable to calcification - although the tropics would have been too hot for coral reefs. See this SkS post: Why were the ancient oceans favorable to marine life when atmospheric carbon dioxide was higher than today?
Please take the time to read and understand the OA not OK SkS series on the topic of ocean acidification. Also, note that further nonsensical comments from you on this subject will likely attract moderation.
aleks, see the series of posts OA Not OK for a more correct chemical explanation that contradicts your assertion that "Chemical calculations show that the effect of CO2 on seawater acidity accepted by the majority of climatologists is highly exaggerated."
Tom Dayton,
Aleks posted a bunch of nonsense on ocean acidification in November, 2017. There are a few posts from him here on the OA is not OK series you referenced. Aleks does not understand ocean carbonate chemistry and cannot understand chemistry when it is explained to him.
Tom Dayton,
Let's consider one example of “correct chemical explanation” in OA is not OK. In the part 12, Fig.6 the graph of pH change in 1990 – 2010 is given, and the drop of pH in 20 years is found of 0.035. The data are processed contrary to the statistics rules without specifying the uncertainty and the correlation coefficient. The scatter of the data during the same year is about 2 times greater than above mentioned drop.
Compare, please, this graph with Fig.13 in part 14. According to Fig. 13, pH value in Atlantic ocean is of 8.1, in Pacific 7.8. In the Fig. 6, pH value about 8.1 is given, while measurements are made on the Hawaii Station.
Can you explain these facts? What about Michael Sweet's explanation: “pH is Pacific is less, because Pacific was formed before Atlantic”?
And the last. It took you 15 minutes to find my post and define it as incorrect. But neither you, nor anyone else, having “the right theory” didn't try to answer Michael Schroeder's question (@76) for more than 2 weeks. May be you will offer your explanation?
[DB] Baiting snipped.
Recommended supplemental reading:
Scientists Pinpoint How Ocean Acidification Weakens Coral Skeletons, News Release, Woods Hole Oceanographic Institute, Jan 29, 2018
I am a 1st year biology student who dreams of becoming an expert in plant biology and organic chemistry at some far off point in the future. First off, I want to thank everyone here for the nuanced and intelligent level of discussion. In reading both the article, and the ensuing discussion posts, I have 2 questions that are probably answered by previous posters but am hoping that you may take time to help me understand more deeply. With increased CO2 in oceans and the ensuing rise in carbolic acid and reduction in the aragonite in the water, how much more energy does it take for coral and plankton to create their exoskeletons? Second question, and this may be stupid, but after past mass coral die offs, how long did it usually take for a rebound or regrowth of coral based on fossil records?
Thank you all again for the lively and intelligent debate despite the fact that at this point, most of this information went over my head. I plan on reading as much as I can on this topic since currently we are learning about chemical reactions in water and also about formation and modification of biological molecules and I find this topic extremely fascinating and also extremely scary and am really interested in gaining a more informed and nuanced understanding of these topics.
Cheers
forgive my foolishness, meant carbonic acid, not carbolic acid
The prediction is that higher atmospheric CO2 will lead to increased ocean acidification from the CO2 forming carbonic acid. And that the higher acidification will interfere with molluscs and crustaceans being able to form hard shells. The shells are fundamentally calcium carbonate CaCO3.
The bit I don't get is that all the limestone deposits in the world which is calcium carbonate were produced when the atmospheric CO2 was many times higher than today. So how did all the shellfish create so much shells that it formed huge limestone deposits with the very high atmospheric CO2 back then??
Markoh @84 , read this thread's OP (both the basic and intermediate form) for some detailed information. You will also find much of interest in the subsequent comments.
The short answer is the combination of acidity & carbonate & bicarbonate balances, with the gradually-evolved capabilities of organisms to produce calcite and/or aragonite structures (bound in organic matrices that are properly suited to the conditions). The rapidity of change in modern ocean chemistry ~ is the big problem. The rapidity of change is outstripping the ability of organisms to evolve to meet the new circumstances. Some organisms do okay, some are adversely affected . . . and the whole ocean ecology worsens (in the "short term" of a few thousand years). It's not just the shell-forming creatures, but the huge pyramid of fish species etcetera resting on the calcium-users.
If you are thinking of purely relevance to humans, then the problem is that we have a huge population ~ and where many have a high proportion of marine diet for protein.
If I may quote from a NOAA fact sheet :-
"Ocean acidification is an often overlooked consequence of humankind's release of carbon dioxide emissions into the atmosphere from fossil fuel burning. Excess carbon dioxide enters the ocean and reacts with water to form carbonic acid, which decreases ocean pH ... and lowers carbonate ion concentrations. Organisms such as corals, clams, oysters, and some plankton use carbonate ions to create their shells and skeletons. Decreases in carbonate ion concentrations will make it difficult to form hard structures, particularly for juveniles. Ocean acidification may cause some organisms to die, reproduce less successfully, or leave an area. Other organisms such as seagrass and some plankton may do better in oceans affected by ocean acidification because they use carbon dioxide to photosynthesize, but do not require carbonate ions to survive. Ocean ecosystem diversity and ecosystem services may therefore change dramatically from ocean acidification."
[my bold]
The second problem : is that we don't yet have a firm idea of how bad it would all get, for humans as well as the ocean ecology. And as the saying goes ~ it would foolish to gamble big-time with Planet-A.
Markoh, I don't know whether you've see it, but there's an old movie "Soylent Green" [a mixture of very good and very "corny"] . . . classic Sci-Fi . . . set in the "near future" ~ grossly over-populated world, food shortages, major civil unrest, deteriorating farmlands (with armed guards). Suicide is almost a patriotic duty. In one of the final scenes, the hero learns a State Secret : the oceans are dying.
That concept was an over-dramatic fantasy, for a 1973 movie. But more worrying, today.
Nice question Mark...
Eclectic @85 But you response skirts around the 1 and only qustion I asked. That is, why CO2 concentrations many times higher than today was not a problem to shellfish when all that limestone was being created!
[JH] Please cite a source or sources for your assertion. Thank you.
Markoh @87,
When I read eclectic's comment @85 I see a clear response to your question and some related additional information.
Maybe you could provide a detailed explanation of why you did not see it that way.
Markoh, first thing about biological systems and climate is that overall, lifeforms can adapt/evolve to a wide range of conditions. There is no "perfect" climate. What is problematic is rapid change - change that occurs faster than adaption can manage.
This applies especially to ocean acidification. Over long timescales (>10,000 years), ocean chemistry is roughly buffered by weathering. Some of the ocean chemistry detail in the "OA is not OK" series. For more about the ocean pH through time, see perhaps this paper.
What the geological record does tell us though is that past rapid ocean acidification events have indeed been a problem. See this recent review especially, chpt 4, "What the past can tell us".
Markoh , the answer is in the reply I gave you. Please read it again, particularly the second paragraph.
It is the combination of ocean chemistry status and the biological evolution of organisms to suit the status quo.
Buffering effects within the ocean, plus the ability of organisms to evolve protein structures that fit their environment. The calcite and aragonite forms of calcium are stabilized/supported by protein matrices, analogous to the way that protein matrices maintain the calcium crystals in your own teeth and bones.
Given enough time, organisms can produce remarkable evolutionary adaptations. Look at the chemistry of single-celled organisms that thrive on the deep surfaces of arctic/antarctic ice, at sub-zero temperatures (at which you yourself would be dead within the hour). At the other end of the scale, are thermophile organisms that thrive in hot springs ~ at temperatures where your own body proteins would be cooked (literally cooked . . . into a frizzle of damaged proteins).
Markoh, evolution takes time to get there. It's the rapid changes which are damaging to individual species and the total ecology of lifeforms.
JH @87 a reference for which assertion Do you mean? That limestone is primarily calcium carbonate or a reference that the worlds limestone was laid down during the time atmospheric CO2 was many times higher than today? as far as I can tell, they are the only assertions I made.
[JH] A reference for the assertion that the world's limestone was laid down during the time atmospheric CO2 was many times higher than today.
Markoh , forgive my bluntness ~ but you seem to be having difficulty in asking a straight question. Is English your first language?
Please put some careful thought into how you frame your question, so that your meaning is clear. Don't rush, but take your time so that you express your underlying concerns about whatever it is that's puzzling you.
@87 , it appears that your assertion is that the high CO2 concentrations in the distant past would have been incompatible with the life cycle of organisms which (ultimately) produced limestone/chalk ~ such as the White Cliffs of Dover. Best, if you cite a source which supports that assertion. But if that was not exactly what you meant, then please re-phrase your comment in a better form. Clarity please !
It is not in dispute that limestone has been laid down when CO2 concentrations are much higher than today (though buffering means that pH was still around 7.5 or higher). See references I supplied further up. It means that organisms have to expend more energy to extrude shell which over long time frames they can adapt to.
However, the issue today is very rapid change in CO2 which results in acidification proceeding faster than organism can adapt and far, far faster than buffering by weathering can ameliorate pH. The paleo record shows this has been a problem for organisms in the past during such rapid excursions, and worse still, CO2 levels may be climbing far faster than in any known previous acidification events. Look for papers on PETM.
JH @87 a reference for which assertion do you mean? That limestone is primarily calcium carbonate or a reference that the worlds limestone was laid down during the time atmospheric CO2 was many times higher than today? as far as I can tell, they are the only assertions that I made.
Eclectic @92 why are you resorting to personal abuse and racism?
My question was very clear. If you don't want to answer it, the good news is that you don't have to.
[PS] Eclectic was out of line but accusations of racism will not further useful discussion. Given confusion by other commentators, your question was apparently not clear to them and so best to clarify rather than perpetuate a misunderstanding.
Markoh @95 . . . personal abuse and racism ?!
WTH are you on about?
Please stop the ridiculous deflections ~ and make your point, if you can. Whatever that mysterious point is !
[PS} Please cool it and stick to science.
Eclectic @96 when you questioned "Is English your first language?", that is casual racism.
[PS] Nope. Many people of same race speak different languages. Drop it. Any further offtopic distractions by either of you will be deleted.
This has all got a bit shouty in a couple of days. Perhaps to return to the initial question @84. Markoh asks:-
I should point out that there is an SkS OP that directly addresses this question (Why were the ancient oceans favorable to marine life when atmospheric carbon dioxide was higher than today?) but perhaps a more succinct answer would be useful here.
Although limestones apparently predate shellfish, shellfish (or molluscs with mineral shells) date back to the Cambrian period when ocean pH was lower than today (perhaps 7.9pH or as low as the 7.3pH modelled by Ridgwell 2005). It is only in the last 30My that ocean pH was high as today (& atmospheric CO2 as low as today). With rising atmospheric CO2, the ocean pH is now falling (today it has fallen from 8.2pH to 8.1pH) and making the chemistry of shellfish more difficult. Those organisms using high--magnesium chemistry (as opposed to argon- or low magnesium-chemistry) will be especially vulnerable as will organisms who do not calsify their shells 'internally', but all will suffer. The last example of CO2 driving ocean acidification (the PETM 55My ago) saw limestones entirely absent from geological formations.
However, it is not the ocean pH that is directly the problem. It is the low concentration of calcium ions that makes shell-formation difficult and such concentrations being pushed low by dropping in pH, not by low pH. Thus over most of the last 500 million years, ocean pH was much lower than today and during these times shellfish thrived.
Even just a slight change in the pH of the ocean can affect it a lot, this includes all the animals living in it as well. As time goes on the amount of CO2 that humans are emitting through industrial activities is increasing, this means the amount of CO2 that the oceans are absorbing is also increasing, if this continues at the rate that it is going, there are going to be extreme impacts on the oceans and ocean life. Coral reefs will suffer affecting all the animals that rely on them, and many humans who rely on the ocean will suffer as well.
Please note: the basic version of this rebuttal has been updated on June 25, 2023 and now includes an "at a glance“ section at the top. To learn more about these updates and how you can help with evaluating their effectiveness, please check out the accompanying blog post @ https://sks.to/at-a-glance
Thanks - the Skeptical Science Team.