Recent Comments
Prev 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Next
Comments 101 to 150:
-
TWFA at 03:09 AM on 4 September 2024New paper about detecting climate misinformation on Twitter/X
It appears [Snipped] the authors would be exellent candidates to be the founding and governing directors of the Ministry Of Truth.
Moderator Response:[BL} It appears that you have unfinished business on a thread where you commented two weeks ago.
Participation in the comments threads at SkS requires that you engage in legitimate, constructive dialog with other participants. You are violating the sloganeering section of the Comments Policy, which states:
No sloganeering. Comments consisting of simple assertion of a myth already debunked by one of the main articles, and which contain no relevant counter argument or evidence from the peer reviewed literature constitutes trolling rather than genuine discussion. As such they will be deleted.
On the previous thread, you made unsupported assertions that have been refuted by other comments. You have provided no response to any of that material. You will not be allowed to start a new thread of unsupported assertions until you go back to that thread and respond to your critics. Suitable responses could include:
- Admitting your errors and agreeing to the corrections.
- Providing additional information and links to scientific evidence that your assertions are supportable.
- Providing more detailed explanations of your positions, and explaining how your original comment was misunderstood.
- etc.
Until you return to that thread and engage in honest discussion, any further comments you post on any other thread will be deleted.
Please note that posting comments here at SkS is a privilege, not a right. This privilege can be rescinded if the posting individual treats adherence to the Comments Policy as optional, rather than the mandatory condition of participating in this online forum.
Please take the time to review the policy and ensure future comments are in full compliance with it. Thanks for your understanding and compliance in this matter.
-
Bob Loblaw at 02:28 AM on 4 September 2024On Hens, Eggs, Temperature and CO2
Mark Johnson @ comment 18:
No, rkcannon @ 16 is not clearly referring to anything specific. For his first comment on this thread, he just comes up with one statement (in two sentences), and can't be bothered to tells us whether he's looking at the original post, or one of the comments? Did he put more than 20 seconds of thought into his question?
Even if we look at the graph in comment 6, which spike is he referring to? Possibly the last one, but he has not take the time to write a clear comment explaining exactly what he is looking at or explain his reasoning. And he has not returned to clarify what he means - which I asked him to do.
rkcannon has a history here. which includes several occasions of throwing out one-liner "gotcha" kinds of questions, and then not bothering top engage in any constructive discussion when his errors are pointed out. That behaviour is "hardly conducive to constructive debate".
And yes, I have read the Koutsoyiannis paper referred to in this post. I have also read the comments on that paper and an earlier one on PubPeer, as I indicated in comment 3. The comment from rkcannon @ 16 comes on the heels of one he made on another thread. In that thread, rkcannon quoted the abstract of another Koutsoyiannis paper (emphasis added):
Recent studies have provided evidence, based on analyses of instrumental measurements of the last seven decades, for a unidirectional, potentially causal link between temperature as the cause and carbon dioxide concentration ([CO2]) as the effect.
Similar statements appear in several Koutsoyiannis papers. The statistical technique used by Koutsoyiannis is incapable of detecting multi-directional processes, and it is incapable of detecting correlations at multiple time scales. Even though Koutsoyiannis et al do not state it or imply it, it is an essential characteristic of their method. The fact that they do not even realize this is why Koutsoyiannis keeps producing papers that contain the same basic error. In essence, they have assumed their conclusion as a result of their methodology.
-
Mal Adapted at 01:20 AM on 4 September 2024Climate - the Movie: a hot mess of (c)old myths!
Aw, hell! Beware of hasty editing. Replace only the first occurrence of "collective" with "individual", not the second. That's my whole point!
-
Mal Adapted at 01:15 AM on 4 September 2024Climate - the Movie: a hot mess of (c)old myths!
Er - y'all can replace "collective" with "individually" in my previous comment, please
-
Mal Adapted at 01:08 AM on 4 September 2024Climate - the Movie: a hot mess of (c)old myths!
Except for the accent, that Australian Senator could have been the late Sen. James Inhofe (R-OK), or Sen. Ted Cruz (R-TX) of the US upper house, i.e. "the world's most exclusive club". Sadly, Rennick's arguments are transparently motivated by his self-interest, like most politicians: "It is difficult to get a man to understand something, when his salary depends upon his not understanding it!". Upton Sinclair's epigram is an English-language commonplace, understood implicitly by all mature climate realists. We know very well that no reality-based case for anthropogenic climate change will ever persuade these guys. We can all enjoy a snicker amongst ourselves at Potholer 54's otherwise-infuriating documentation of political craft, but the only collective individual action most of us can realistically take is to try to ensure he's not re-elected.
You Australians will have to handle that problem yourselves, I'm afraid. Here in the US, I'm taking heart from the latest Six Americas report from Yale's Climate Change Communications program. Americans who were "alarmed" or "concerned" about climate change have gone from minority to majority since 2013, while those who were "disengaged", "doubful" and "dismissive" fell from 33% to 28%. The number of us who were "cautious" ten years ago has fallen from 26% to 15%. Heh. "Cautious", hell! Any of us who lived through the PNW heat dome of 2021, for example, have seen the wiser side of caution: namely collective action to cap global warming. I can't otherwise account for the changes over time, but they surely must be in Kamala Harris's favor, no?
Moderator Response:[BL] Edited as requested (I think) - although individually we can only vote once, and election results represent collective action.
-
Mark Johnson at 01:01 AM on 4 September 2024On Hens, Eggs, Temperature and CO2
Bob Loblaw @ 17:
He's clearly referring to the graph in post 6, not figure 6. It took me 20 seconds to realize this - perhaps you could have taken 20 seconds of your own time before jumping down his throat?
And by the way, his question is a reasonable one and deserves a civil reponse. Using language like 'what on earth...?', 'are you seriously thinking..?', '..some tiny feature that you think..' are hardly conducive to constructive debate.
And if you'd actually read Koutsoyiannis et al, you would know that they absolutely do not say or even imply 'that there is only one cause in one direction at all time scales'.
-
nigelj at 07:21 AM on 3 September 20242024 SkS Weekly Climate Change & Global Warming News Roundup #35
The mantra is dont worry about climate change because when it gets bad we will find great technical solutions. To me its like saying don't worry about smoking tobacco because by the time you get sick, there will be excellent treatments. Yeah sure there will be. Its 100% guaranteed.
-
Bob Loblaw at 05:05 AM on 3 September 2024Climate - the Movie: a hot mess of (c)old myths!
Yes, another good video from Potholer54. I think I share his sarcastic sense of humour - and at some point as you look at this level of denial from a politician you have to make a choice between crying and laughing at it.
I am currently reading Authoritarian Nightmare - Trump and his Followers, by John Dean and Bob Altemeyer. (Yes, John Dean of Watergate fame). Much of the social psychology it discusses is from Bob Altemeyer's career studying what he termed authoritarian follower psychology. You can access much of Altemeyer's material (including an ebook The Authoritarians), at https://theauthoritarians.org/.
Dean and Altemeyer discuss two psychological aspects of the extreme devotion to the sort of politicians' messages exposed by Potholer: they refer to "social dominators", who treat every interaction as a situation where they need to dominate others (or other groups), and authoritarian followers, who are highly susceptible to being led down the garden path by authority figures. When someone comes along that tells them what they want to hear, they lose any ability to critically evaluate what they are being told. Being lied to isn't a problem as long as the message confirms what they deeply want to believe.
Large numbers of authoritarian follower voters and lying power-hungry demagogue politicians is not a good mix.
-
Bob Loblaw at 04:42 AM on 3 September 2024On Hens, Eggs, Temperature and CO2
rkcannon @ 16:
What on earth are you talking about? Figure 6 does not have temperature in it, so what temperature graph are you referring to? And figure 6 is showing short-term variation that includes annual or shorter times. It is a rate of change graph, not a cumulative storage graph. And which "very high spike" are you referring to? What year?
...and are you seriously thinking that every temporary spike in CO2 (really, rate of CO2 change, if you are using figure 6) will lead to a temperature spike? It takes time for global temperatures to change. The atmosphere alone has a sufficiently large heat capacity that it takes months for an energy imbalance of a few W/m2 to reach a new equilibrium. If you take the shallow ocean mixed layer (<100m depth), the heat capacity means it take a decade or two for it to adjust. When you take the deeper ocean into account, the time lag increases proportionally.
...and CO2 is not the only factor affecting temperature. especially on the shorter time scales.
By thinking that every little short-term spike in CO2 has to correlate with a spike in temperature, you are making exactly the sort of basic error that Koutsoyiannis et al have made (several times). Koutsoyiannis has assumed that there is only one cause in one direction at all times scales, and has completely ignored the multi-factor and multi-time-scale nature of the carbon cycle and global temperature - and done it using a technique that removes the multi-decade slow rise in CO2 and how it correlates with global temperature.
It seems that you are not looking at the whole system, and focusing narrowly on some tiny feature that you think disproves the big picture.
-
rkcannon at 03:29 AM on 3 September 2024On Hens, Eggs, Temperature and CO2
Why don't we see a higher temp spike along with the very high CO2 spike in the graph in 6? It seems CO2 is not influencing T at all.
-
One Planet Only Forever at 03:21 AM on 3 September 2024Climate - the Movie: a hot mess of (c)old myths!
Eclectic (and John Mason),
Thanks for pointing to the entertaining and informative Potholer54 video.
I have an opinion to share regarding the claims that Rennick makes on his official web-based communications” (that I consider to quite likely be a correct understanding)
- On the header band of his official web page (shown at 4:50, and many other times, in the video) he claims to be “Working for all Australians”
- On his self-promotion image at 15:15 (Just before the shift into ‘The opinion room’) Rennick claims “What binds us together is much more than what drives us apart”.
Those two claims make sense if:
- the 'us' in the second claim is the diverse collective of anti-intellectual resisters of learning who appreciate that they have power if they vote together for their extensive diversity of deliberately conserved misunderstanding and associated deliberate limited awareness.
- 'Australians' in the first claim is restricted to the sub-set of anti-intellectuals in Australia.
A significant portion of the population in many nations undeniably collectively vote for leadership that aligns with their interest in prolonging, and attempting to increase the popularity of, misunderstanding and the related required limited awareness.
It seems that Senator Rennick is one of the many anti-intellectual leadership candidates who consciously chooses to ‘work’ on ways to continue to get as many of the diversity of anti-intellectual resisters of learning to vote for him rather than driving away voters who choose to passionately ‘resist learning’.
-
Bob Loblaw at 02:26 AM on 3 September 2024CO2 emissions do not correlate with CO2 concentration
rkcannon:
An earlier paper by Koutsoyiannis was debunked on this post here at SkS. He has been repeating the same basic bogus analysis in a series of papers, all of which are basically junk.
In the snippet you copy (which appears to be the start of the abstract), he again repeats his assertion of "evidence ... for a unidirectional, potentially causal link between temperature as the cause and carbon dioxide concentration ([CO2]) as the effect." This claim appears in most (all?) his previous works, and it is still "not even wrong".
I notice that Koutsoyiannis has again chosen an MDPI journal for his assertions. MDPI does not have a particularly good reputation, having been a go-to location for a lot of bad papers that do not get proper review.
Is there any reason to think that Koutsoyiannis has actually gotten something right this time? For the most part, papers by Koutsoyiannis are simply not worth reading.
-
rkcannon at 01:35 AM on 3 September 2024CO2 emissions do not correlate with CO2 concentration
Link to previous comment
https://www.mdpi.com/2413-4155/6/1/17
-
rkcannon at 01:34 AM on 3 September 2024CO2 emissions do not correlate with CO2 concentration
Regarding C14, the gradual decline may be due to the nuclear tests that created C14 that is decaying or disappearing naturally. Ref this paper. Also this paper also looks at 13C/12C ratio, saying the following. Is there discussion on this somewhere?
Net Isotopic Signature of Atmospheric CO2 Sources and Sinks: No Change since the Little Ice Age
by Demetris Koutsoyiannis
[ORCID]
Department of Water Resources and Environmental Engineering, School of Civil Engineering, National Technical University of Athens, Heroon Polytechneiou 5, 157 72 Zographou, Greece
Sci 2024, 6(1), 17; https://doi.org/10.3390/sci6010017
Submission received: 19 December 2023 / Revised: 23 February 2024 / Accepted: 29 February 2024 / Published: 14 March 2024
Abstract
Recent studies have provided evidence, based on analyses of instrumental measurements of the last seven decades, for a unidirectional, potentially causal link between temperature as the cause and carbon dioxide concentration ([CO2]) as the effect. In the most recent study, this finding was supported by analysing the carbon cycle and showing that the natural [CO2] changes due to temperature rise are far larger (by a factor > 3) than human emissions, while the latter are no larger than 4% of the total. Here, we provide additional support for these findings by examining the signatures of the stable carbon isotopes, 12 and 13. Examining isotopic data in four important observation sites, we show that the standard metric δ13C is consistent with an input isotopic signature that is stable over the entire period of observations (>40 years), i.e., not affected by increases in human CO2 emissions. In addition, proxy data covering the period after 1500 AD also show stable behaviour. These findings confirm the major role of the biosphere in the carbon cycle and a non-discernible signature of humans. -
pattimer at 22:27 PM on 2 September 20242024 SkS Weekly Climate Change & Global Warming News Roundup #35
It's undoubtedly true that we need to both face denial and how to create solutions ( both at government/ national level and at the individual level). Sceptical science does a superb job at rebutting denial. I also think denial is part of the continual procrastination process and many of those such as the GWFP that have played a huge role in denial of climate change have changed their procrastination strategies. It seems that they will make political gain by accepting long term strategies for climate action giving us "world leading targets"which they will then make further political gain by reneging on them when the time comes to implement them. Another strategy is raising false hopes that are completely unrealistic to delay implementing existing strategies such as implying that our boilers and cars in the future will have hydrogen delivered along pipes. This they know will make people delay personal changes that would otherwise reduce their fossil fuel consumption. So we not only need to face denial of the climate science but also the denial of solutions that could be presently implemented. I personally arrange regularly visits to my home by small groups of individuals to address the myths about heat pumps for example that the procrastinators have been successful in flooding social media with in the same way they spread climate denial.
-
John Mason at 17:31 PM on 2 September 2024Climate - the Movie: a hot mess of (c)old myths!
@121:
Link to Potholer 54's video:
https://www.youtube.com/watch?v=lBV5fw6e0RM -
Eclectic at 04:16 AM on 2 September 2024Climate - the Movie: a hot mess of (c)old myths!
For your general amusement :-
Another Potholer54 climate video, posted 31st August 2024, and so far getting 40,000 views and 2,000 comments (many quite witty).
Title :
"Could this be the stupidest politician in Australia?"
Yes, the USA does not have a monopoly.
Video 18 minutes long.
"Read 'em and weep."
-
Eclectic at 21:13 PM on 30 August 2024Climate Adam: Can Coral Reefs survive Climate Change?
Er .... the GBR [Great Barrier Reef] has never been better than now?
Rose-tinted glasses & cherry-picking are probably not the responsible and sensible approach to assessing and managing the GBR.
Heat (and pollution?) will certainly increase over coming decades, for reasons which you already know. So the GBR corals are facing a long uphill battle.
Perhaps the evolution of greater heat-resistance in many coral types (and their symbiotic algae) will occur ~ but will it be fast enough to preserve the majority of the GBR in a diverse form, or will the GBR deteriorate into something approaching a sad "monoculture"?
Maybe that sort of "recovery" of the GBR will occur in the matter of a decade ~ or perhaps there may be a large "valley of death" until evolution catches up. A lot is uncertain . . . but the corals are definitely facing a long uphill battle against the increasing heat.
MadMackz, your views may have been influenced by your innate cheerful & optimistic nature ~ reinforced by the outlier views of Dr Peter Ridd-Micawber and the false smiles of the GBR tourist boat operators.
-
MadMackz at 11:08 AM on 30 August 2024Climate Adam: Can Coral Reefs survive Climate Change?
The only issue I have with this is that it doesnt really promote the things that people really should also know about coral reef namely the Great Barrier Reef. It is strange how although through the mass events that have killed off so much precious reef, that since 2022 we have observed Record Highs regarding size, growth and overall health of the GBR in all recordable history in its entirety. 3 years in a row! Which is great! Something else that many people dont know is that although record levels of the reef have been observed there has been a detrimental outbreak of starfish which are one of the largest offending predators of the reef, them along with Fish, marine worms, barnacles, crabs, snails and those pesky sea stars all prey on the soft inner tissues of coral polyps. But that still is not the largest Killer of these beautiful reefs. That...would be waves, from cyclones and storms.. Breaking off large pieces of coral at a time and literally shredding it as the waves flatten it to litteral sand. Its a good thing that those cyclones have been showing a decline in frequency and intensity for the last half a decade. One plus of the warm waters though is the spawn of new reef is increased, Once a year, on cues from the lunar cycle and the water temperature, entire colonies of coral reefs simultaneously release their tiny eggs and sperm, called gametes, into the ocean. The phenomenon brings to mind an underwater blizzard with billions of colorful flakes cascading in white, yellow, red, and orange.
In ways that scientists still do not fully understand, mature corals release their gametes all at the same time. This increases their chance at diversity and has shown the mixing of genes to increase their tolerance to the changing climate and temperatures. And finally while individual coral colonies suffer from a degree of bleaching in any given summer. This is a natural process and not of particular concern, what we do know is the reef has never been better than now and heres to another 3 years of Great Barrier Growth.
https://www.youtube.com/watch?v=QnQPSYC3IdI
https://www.youtube.com/watch?v=znOidiyUnq8
I want to note that there are sources out there claiming widespread bleaching events are killing off the reef . Namely ABC New3s Australia, I will note that Comments are all turned off, and I suggest seekingout the words of those that work with the reef everyday, Give them a call, go take a tour. Get out and Get the Facts for yourselves, If it matters , Its worth it, and you deserve it. -
Jeff Cope at 13:11 PM on 28 August 2024CO2 is just a trace gas
Put some table salt in your hand and dump out all but 2 grains. Keep hold of them while you read this:
Cyanide’s LD50, the dose that kills half those exposed to it, is 13 parts per million. Arsenic’s is 6. Some snake venoms, algal bloom toxin, & ricin, can kill at 1 part per million (0.0001%).
The main source of methyl mercury in the world is coal burning. It can kill in quantities smaller than 1ppm; at even smaller doses it doesn’t kill, it just has scores of profound lifelong physical and mental effects. Are there Alice in Wonderland fans here? Alice’s Hatter spoke in the bizarre tangles called word salad as a result of what’s Put some table salt in your hand and dump out all but 2 grains. Keep hold of them while you read the next 2 paragraphs.
Cyanide’s LD50, the dose that kills half those exposed to it, is 13 parts per million. Arsenic’s is 6. Some snake venoms, algal bloom toxin, & ricin, can kill at 1 part per million (0.0001%).
The main source of methyl mercury in the world is coal burning. It can kill in quantities smaller than 1ppm; at even smaller doses it doesn’t kill, it just has scores of profound lifelong physical and mental effects. Are there Alice in Wonderland fans here? Alice’s Hatter spoke in the bizarre tangles called word salad as a result of what’s still called Mad Hatter syndrome. It takes even less than that 1ppm in the chronic doses hat makers breathed in from the mercury nitrate (Hg(NO₃)₂xH₂O) they used for about a century to toughen fur fibers, allowing them to matt together better for a firmer hat. The process was called carroting because Hg(NO₃)₂xH₂O is orange.Phyllobates terribilis, Golden Dart Poison Frog, ‘Orange’ (Imagine a picture here)
Dart poison frogs' batrachotoxin's LD50 is 1/1000th of that (1mcg/kg or 1 part per billion), so if those 2 grains of salt in your hand were batrachotoxin, it's a coin flip whether it would be enough to kill you just by holding it, if you had even a tiny cut on your palm. Sorry, there’s no antidote, and you have about 9 minutes left. It would take 6 salt grains worth of VX, while Botulinum toxin kills at 1 thousandth that amount—1 nanogram, or 1 billionth of a gram/kg body weight. One part per trillion of it can kill.
9 ppm is the maximum indoor safe carbon monoxide level over 8 hours. 200 ppm or greater will cause physical symptoms and is fatal in hours. 800 ppm of CO or greater in the air is fatal within minutes.
-
Christian Moe at 21:33 PM on 26 August 2024It's cosmic rays
PS to comment 123: Apart from the ion/muon confusion, I'd suggest that human radiation doses are a digression that is apt to confuse the reader as to what the issue is here, especially in a short at-a-glance section.
-
Christian Moe at 20:59 PM on 26 August 2024It's cosmic rays
The current "at a glance" section, second paragraph, appears to confuse ions with pions and muons:
"When cosmic rays hit the top of our atmosphere [...] they interact with the atoms up there producing showers of charged particles known as ions. The ions then head on down towards the surface, where they make up just over ten percent of our typical yearly radiation dose. That's approximately equivalent to three chest x-rays."
In my understanding, cosmic rays hitting atoms at the top of the atmosphere produce pions, charged pions decay into muons, which continue down the atmosphere, creating ions as they pass. Ions are charged atoms or molecules. Ions do not (as such) contribute to your radiation dose. Ionizing radiation does (like muons and the rest of the cosmic-ray cascade products).
-
scaddenp at 09:36 AM on 23 August 2024What should you do to prepare for the climate change storm?
Oceanfront is fine - if you are on hard rock a few metres above max high tide level. Beachfront - not so much. Soft sediment erodes easily in storm surges and of course, rising tide. TWFA - what data about sealevel rise happening right now are you disputing is true? What data is the basis for your predictions?
-
One Planet Only Forever at 09:29 AM on 23 August 2024What should you do to prepare for the climate change storm?
A point regarding TWFA's opinion about people abandoning Manhattan:
Bloomberg reports on "New York City’s Basement Residents Face Financial Risk of Floods"
Parts of Manhattan may be 'abandoned'.
-
One Planet Only Forever at 08:40 AM on 23 August 2024What should you do to prepare for the climate change storm?
Nigelj @2 and Michael Sweet@3,
Thank you for researching and sharing the evidence that clearly contradicts TWFA’s poorly justified opinion that “in our lifetimes ... nobody will have abandoned ... their beachfront homes or estates due to climate”.
Based on a short time doing unbiased investigation and thoughtful consideration I offer the following additional evidence contradicting or weakening TWFA’s poorly justified opinions expressed @1:
- many island nations, like the Maldives, are already suffering destruction and forced escape/migration due to climate change impact induced sea level rise.
- The feet of the Statue of Liberty are more than 150 feet above current sea level ... so ... weird statement.
- The Battery Park City Authority is planning major costly mitigation actions (BPC NEW LOOK: STORM SURGE BLUES link here) to try to ensure that “Battery Park will still be there”. Those mitigation/adaptation actions are a costly distraction that would not have been needed if climate change impacts had been reduced sooner with a lower level of peak total impact like 1.0 C achieved. If those mitigation were not required then all that money/effort could have been expended to genuinely improve the future of Battery Park.
- The Obama homes at the Vineyard and Hawaii both appear to be situated high enough to not be at risk of storm surge damage “during our lifetimes” even if ‘our lifetime’ is the 100 year lifetime of a new born today, as long as global warming impacts are limited to 2.0 C. Of course, if ‘our’ is regarding humanity’s lifetime of potentially many millions of years then those homes are likely not high enough.
A final note: Warren Buffet’s actual statement and the context of it are well presented by Investopedia here. Not quite what TWFA opined.
-
michael sweet at 08:16 AM on 23 August 2024What should you do to prepare for the climate change storm?
I visited the outer banks in North Carolina 18 months ago. We stayed in a house on third Street. It was one and a half blocks to the beach. First Street and half of second street were gone.
This week this video was widely on mainstream news of another house there falling in the ocean. I saw a community on the Chesapeake Bay where the houses were uninsurable and a fishing island there is trying to get the feds to spend millions to prevent their island from washing away (a hopeless task).
I saw a video on YouTube of farmers in Vietnam who used to raise rice and now grow salt water fish and crabs. Another foot of sea level rise will overtop their levies and they will become refugees.
The lowest houses are beginning to be washed away world-wide. Insurance rates in Florida and other USA beach front are artificially held down by the government.
-
nigelj at 07:02 AM on 23 August 2024What should you do to prepare for the climate change storm?
Climate change could indeed make properties uninsurable and hard to sell. From stuff.co.nz:
"Homes on parts of New Zealand’s coast will begin losing access to affordable insurance within 15 years, according to a stark new report. Wellington will be hit first, and Christchurch hardest, but all four major cities will be affected, according to new research led by climate and insurance specialist Belinda Storey for the Deep South National Science Challenge.By 2050, at least 10,000 homes in our biggest cities will be effectively uninsurable, however spiking premiums and policy exclusions could start being felt as soon as a decade from now, it concluded."
"In Wellington, just 12cm of sea level rise could see average premiums more than quadruple for about 1700 homes, the report estimates – if insurers fully priced the increased risk into policies. At those levels, people may effectively find they have no insurance cover, said Storey."
Ten to fifteen years is not far away. So it may pay to research the risks in your area now, and sell well before you suddenly find insurance is unavailable. I would say that when insurance companies start increasing premiums to high levels, or refusing cover at any price it could also happen suddenly without much warning. Decision making ruminates away for a long period then reaches sudden tipping points like other things in life..
-
TWFA at 23:26 PM on 22 August 2024What should you do to prepare for the climate change storm?
Head for the hills! Warren Buffet said the time to be scared is when people are greedy, and the time to be greedy is when people are scared, so it sounds like oceanfront property should become a bargain once the scared migrate to Michigan. My own prediction is that in our lifetimes the Statue Of Liberty will not have wet feet, Battery Park will still be there and nobody will have abandoned Manhattan or their beachfront homes or estates due to climate, it will just be wealthier folks living there, like the Obamas with their oceanfront estates in the Vineyard and Hawaii, with an additional two climate escape pods in D.C and Chicago.
-
Bob Loblaw at 00:57 AM on 21 August 2024Are climate models overestimating warming?
ubrew12:
As MA Rodger says, climate models do include soil moisture and surface albedo. The surface component of these models will also include vegetation cover, as this strongly influences the evapotranspiration rates. This is an essential part of the climate modelling process, as the surface energy balance has major implications in partitioning energy within the climate system.
The surface energy balance involves:
- solar radiation reaching the surface,
- IR radiation emitted from the atmosphere to the surface,
- IR radiation emitted from the surface to the atmosphere,
- energy transported as "sensible" heat (temperature) between the surface and the atmosphere (on average, upward)
- energy transported as "latent" heat (evapotranspiration, condensation) between the surface and the atmosphere (on average, upward, representing water movement from the surface to the atmosphere)
- energy transported via the conduction of heat between the surface and the subsurface (soil or water).
The concept of a "surface energy balance" is based on the idea that the surface is an infinitely thin plane that separates the atmosphere and the earth (land/sea). With no thickness, it has no mass, so it cannot store energy. There must be an energy balance that sums to zero for all energy flows to or from the surface. In this concept, the land itself is the sub-surface (which can store energy).
NCAR has a good web page describing their models. The overall climate model is built from several components: atmosphere, land, ice, etc. For the land component, the docuimentation table of contents lists (under "special cases") things like "Running the prognostic crop model" and "Running with irrigation".
So yes, it is possible to run these models with various aspects of surface conditions. Whether anyone has is another question - and getting appropriate historical surface data to do so accurately is an even bigger question.
-
MA Rodger at 02:08 AM on 20 August 2024Are climate models overestimating warming?
ubrew12 @3,
The models do certainly calculate soil moisture and account for surface albedo. I don't know how accurately this is done. Presumably, if it were done badly enough to affect the modelling generally, such a failing would be quickly corrected.
You ask this because you wonder whether the 'Dust Bowl' could be the reason for these Corn Belt states having seen such low warming rates 1973-2022. Perhaps they began the period with warming already in place.
The GISTEMP web site easily allows such ideas to be tested. Over the full 1880-2022 period of data, the same low warming trend is still seen across the eastern USA thro' summer months on a global map. It is actually there all year and strongest in Autumn,weakest in Winter & Spring. So using this region to be representative of AGW, it is simply a dishonest cherry-pick (which is what 'Derwood Turnip' is doing). And as a region testing the climate models, as shown in the global map above in the OP, it is again a dishonest cherry-pick (which is what Roy Spencer is doing), although Montana/North Dakota would give a more dramatic result, indeed the most dramatic result.
-
ubrew12 at 21:58 PM on 19 August 2024Are climate models overestimating warming?
This article includes a graph of the worlds 1970-2023 prediction anomaly. This is pure speculation, but the anomaly in question may not be simply 'unforced variability'. We know that in the 30 years before 1970, the Corn Belt was recovering from the 'Dust Bowl': non-evaporative fallow land was being replaced by irrigated crops. Post 1970 this trend would have continued, as better agricultural practices filled the summer Corn Belt with evapotranspirating crops: a form of human agency the climate models may not include as a boundary condition. If so, then such a overprediction anomaly may also be found in other cropland areas, like in Ukraine.
An opposite effect might be expected in places where evapotranspirating jungle was, post 1970, being cut down and replaced with relatively inefficient ranchlands, soybeans, and palm oil plantations: Brazil and Borneo. Hence, they show up colored blue in that graph.
I'm just speculating. Do the climate models account for this kind of human agency, land-use change, as a boundary condition?
-
MA Rodger at 20:40 PM on 19 August 2024Are climate models overestimating warming?
ubrew12 @1,
Your friend should rest assured that his crystal ball, ouija board and morning cuppa are all safe. Even Foghorn Leghorn can sleep easy in his bed. The fake human 'Derwood Turnip' obtains the best divinations ever in history and he uses other means.
In the case of the corn state summer tmperatures, there is a bit of a disconnect between 'Derwood Turnip' and the information he presents. The actual author is the blunderful denialist Roy Spencer who posted an analysis on his blog in June 2023 and then included it in a pack of nonsense he had published in January 2024 by a bunch called The Heritage Foundation. It took 'Derwood Turnip' eight months to re-post the published graphic.
Such delay is something 'Derwood Turnip' has a history of creating. An example of a shorter 82-hour delay 'Derwood Turnip' created back in 2019 involved Hurricane Dorian, initially a Cat-5 hurricane but soon to be dropping to Cat-2. 'Derwood Turnip' used his position as POTUS and his very own exceptional analytical skills to give warning to the good citizens of Alabama (and others) that they "will most likely be hit (much) harder than anticipated" by Hurricane Dorian which was "looking like one of the largest hurricanes ever."
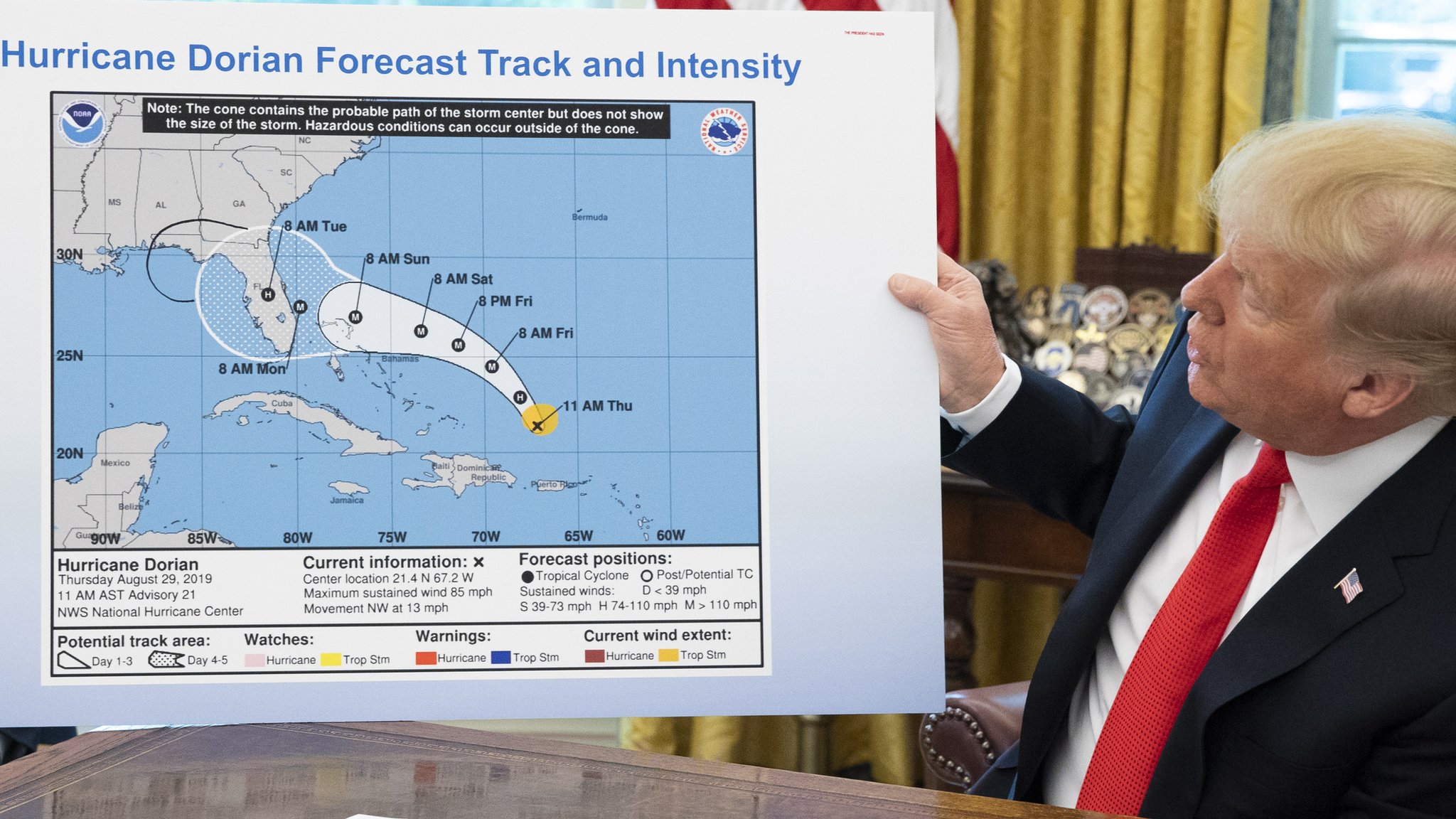
Yet during this 82-hour delay, the situation with Hurricane Dorian had changed dramatically. Advisory #021 had been updated multiple times (as is normal, with updates 4-times-a-day), having been superceded by Advisory #032A three hours prior to the warning of 'Derwood Turnip'.
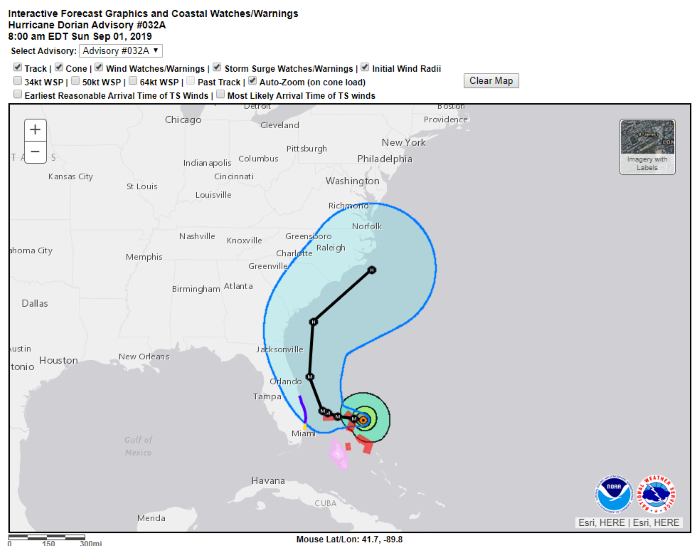
The enormity of the wisdom of 'Derwood Turnip' can be seen in his detailed explanation for the remarkable variance between his wondrous analysis and the reality he so often wrestles with.
Trump, Sept. 4: I know that Alabama was in the original forecast. They thought it was get it — as a piece of it. It was supposed to go — actually, we have a better map than that, which is going to be presented, where we had many lines going directly — many models — each line being a model. And they were going directly through. And, in all cases, Alabama was hit — if not lightly, in some cases pretty hard. Georgia, Alabama — it was a different route. They actually gave that a 95 percent chance probability.
It turned out that that was not what happened; it made the right turn up the coast. But Alabama was hit very hard, and was going to be hit very hard, along with Georgia. But under the current, they won’t be.
-
ubrew12 at 15:52 PM on 19 August 2024Are climate models overestimating warming?
Derwood may be put into a position to make climate policy. If not computer models, what predictive tool was he planning to use to evaluate that policy before implementing it? Crystal Ball? Ouija Board? Tea leaves? Chicken bones? Asking for a friend.
-
prove we are smart at 00:05 AM on 15 August 2024Climate Adam: Kamala Harris and Climate Change - Hope or Hype?
Well, it certainly needs all that because to be the worlds "good" policeman, you need at least 100million barrels of oil a year. It's a bit of an estimate since to disclose your militaries emissions is an optional answer at the COPs. The worlds militaries account for maybe 5.5% of the worlds CO2 in a year. www.aljazeera.com/news/2023/12/12/elephant-in-the-room-the-us-militarys-devastating-carbon-footprint
I certainly agree the classic ugly american Trump is unbelievably bad for most and the planet but as in my Australia and many countries, for many reasons,trust in our chosen public officials has declined and seems the sad new norm. commons.wikimedia.org/wiki/File:Public_trust_in_government.webp
-
RedRoseAndy at 22:18 PM on 14 August 2024Can we air condition our way out of extreme heat?
Heat pumps can be reversed on hot days to cool houses.
-
RedRoseAndy at 22:14 PM on 14 August 2024Climate Adam: Kamala Harris and Climate Change - Hope or Hype?
The US is now the leading producer of fossil fuel in the world. This needs addressing.
-
nigelj at 08:36 AM on 14 August 2024Climate Adam: Kamala Harris and Climate Change - Hope or Hype?
I thought that was an exceptionally well presented video. Its useful to look look at the priorities Trump and Biden place on using tax payers money, spending and borrowing. Trump prioritised tax cuts mostly for already profitable corporates and for millionaires, creating a big deficit, while Harris supported the inflation reduction act that helps solve environmental problems, creates jobs with all skills levels, so benefits a wide group of people, and builds important infrastructure. You choose.
-
RedRoseAndy at 20:33 PM on 13 August 2024Climate change is making us sick, literally
As this is your first post, Skeptical Science respectfully reminds you to please follow our comments policy. Thank You!
-
RedRoseAndy at 20:33 PM on 13 August 2024Climate change is making us sick, literally
We should be biocharing all organic waste to store it as a soil improver in the ground for thousands of years. This would help clean up our rivers and seas.
-
Eclectic at 19:59 PM on 11 August 2024Climate - the Movie: a hot mess of (c)old myths!
BaerbelW @119 :
Many thanks for the link.
Potholer54's science videos are outstandingly excellent in debunking of climate myths (and evolution myths).
Also with some dry humor. And it is a pleasure to see his engagement with the trolls & cranks in the comments sections under his videos. (He despatches them with admirable skill and suavity ~ I have never yet seen him bested in these little contests. It is worth occasionally re-visiting his videos' comments sections, for the entertainment and the instructive value of seeing a master at work.)
This, his latest video, has over 40,000 views in less than 2 days.
-
BaerbelW at 17:48 PM on 11 August 2024Climate - the Movie: a hot mess of (c)old myths!
Eclectic @118
This link may clarify things.
On another note, I just updated the blog post to embed a new video published by potholer54 on August 10, 2024. It's a 37 minute long debunking of the "movie".
-
Eclectic at 04:31 AM on 11 August 2024Climate - the Movie: a hot mess of (c)old myths!
Dikran Marsupial @117 :
It's unclear whether you refer to the OP or to Bob's @116.
If the latter ~ then the names Soon, Koonin & Lomborg . . . may show a form of Nominative Determinism, in that they have two zeroes, which nicely represent the validities of their climate arguments ;o)
(Hoping I haven't managed make a faux pas there, Dikran, for I have forgotten your true name ! )
-
Dikran Marsupial at 00:41 AM on 11 August 2024Climate - the Movie: a hot mess of (c)old myths!
... but apart from that it is O.K.? ;o)
-
One Planet Only Forever at 01:54 AM on 10 August 2024What Project 2025 would do to climate policy in the US
Thank you for sharing this item. It’s a great supplement to the Story of the Week in “2024 SkS Weekly Climate Change & Global Warming News Roundup #29”
Doug Bostrom’s observation about the selective secrecy of the politically conservative collective pursuing Project 2025 is a justified concern.
Harmful exploitation of flaws and weaknesses in the US Constitution is possible and can be very damaging. The US Constitution has been proven to be open to biased poor judgment interpretations. An example is that the Constitution can be interpreted to never require the Senate to vote to approve Supreme Court (SC) nominees. The death of SC Justice Scalia on February 13, nearly 9 months before an election, did not require the New Right Republican Senate to vote on the President’s nominee replacement. But the death of SC Justice Ginsburg on September 18, less than 2 months before an election, resulted in the New Right Republican Senate expediting voting to appoint a new SC justice.Exploitation of that systemic flaw shows how the freedoms and fairness of democracy can only survive if rational judgments govern and the institutions that make a socioeconomic system work as a democracy are defended against irrational influence.
The secrecy regarding the “fourth pillar” of Project 2025 is a serious concern given the following ‘open declaration’ in the item linked to by Doug:
“The 2025 Presidential Transition Project has convened the conservative movement in support of the ideas that will reclaim our nation.”
The New Right Republicans behind Project 2025, including Trump in spite of his denial, do not consider the USA to be a nation for anyone other than ‘their type of people’. By saying “reclaim” they imply that the majority of the current US population is a threat to ‘Their New Right Nation’.
As noted by nigelj @2, Project 2025 is understandably a collective of poorly justified passionately held emotion-based anti-intellectual opinions that conflict with ‘better judgment based on unbiased investigation and thoughtful consideration in pursuit of learning to be less harmful and more helpful to Others’. However, it is well understood that many people can tragically be tempted to passionately fight to embrace and preserve emotion-based opinions, regardless of their ability to learn that they are harmfully incorrect.
Many people who understand the importance of rapidly ending climate change impacts are likely to vote against that rational understanding because of a more powerful harmful desire for Other emotion-based opinions excusing poorly justified harmful Interests.
-
nigelj at 08:05 AM on 9 August 2024What Project 2025 would do to climate policy in the US
Project 2025 seems to be just a wish list, and is full of evidence free assertions. Hitchens Razor (0ne of the philosophical razors) says " what can be asserted without evidence can also be dismissed without evidence".
-
MA Rodger at 22:43 PM on 8 August 2024On Hens, Eggs, Temperature and CO2
Keith R @14,
You are correct that the Koutsoyiannis paper does not assess the on-going +2ppm/yr of CO2 resulting from fossil fuel use (less the ocean and biosphere draw-down).
But your comment has goaded me into a back-of-fag-packet assessment of how much out-gasing the wobbles in the global temperature record would actually achieve. (I'd reckon beforehand it would be exceedingly tiny.)
If the last ice age saw CO2 rise by about 100ppm and global temperature rise about +4ºC, that would suggest a big El Niño-induced temperature rise of +0.4ºC would see CO2 rise 10ppm but only if equilibrium was achieved (which would take about a millenium).
An El Niño temperature wobble is up-&-down in a single year so there is of course no equilibrium. The out-gasing would be greater earlier and quickly tail off: say 50% happening in the first century, so 5% in the first decade and 0.5% in the first year? That would suggest an out-gasing CO2-rise resulting from a +0.4ºC temperature rise in temperature following a big El Niño of just 0.05ppm.
The actual CO2 wobbles the Koutsoyiannis paper relies on being temperature-induced out-gasing (and not drought-induced reductions in forest growth) are about 1ppm. So "exceedingly tiny" is a good description. -
Keith R at 20:51 PM on 8 August 2024On Hens, Eggs, Temperature and CO2
Here’s a simple way to look at the Koutsoyiannis paper… It claims that the data from 1980-2019 has correlations demonstrating that temperature changes drive CO2 and no correlation supporting CO2 driving temperature. He then concludes that this proves CO2 changes can’t drive temperature changes. There is a leap here from not seeing an effect in a 39-year period to the conclusion that it doesn’t happen.
The temperature drives CO2 side of the relationship occurs when something else such an El Nino, solar cycles, etc. causes a temperature change which causes a change in the degassing rate from oceans and that causes CO2 levels to change. These events occurred during the 39-year period and are shown in the Koutsoyiannis analysis.
The CO2 drives temperature side of the relationship occurs when something causes the CO2 concentration to change which modifies the strength of the greenhouse effect and this changes the temperature. Humans were emitting CO2 at a steadily increasing rate during the 39-year period and it is not clear that there were any changes in the rate of CO2 emissions that were significant enough to cause the temperature shift that would be detectable by Koutsoyiannis.
The mechanism used in the paper to look at shifts in the moving difference between values and the previous 5-year average will not detect a steadily increasing CO2 concentration causing a steadily increasing temperature. The paper is focused on the cause-and-effect of shorter-term fluctuations. The paper only shows that all the short-term changes during the monitoring period were the result of temperature changes driving CO2. The claim that this proves CO2 doesn’t drive temperature is unjustified. -
Doug Bostrom at 13:39 PM on 8 August 2024What Project 2025 would do to climate policy in the US
It's notable that the Heritage Foundation is keeping details of the so-called "fourth pillar" of Project 2025 a closely held secret.
Although we cannot identify its exact composition, we can think of the fourth pillar as a cylinder of compressed public policy gas to quickly fill the vacuum created by an incoming president with no interest in or knowledge of public policy.
With no organic competence in forming an administration, this person is an ideal pipeline for delivery of the Heritage Foundation's crafted payload. A uniquely dangerous person but ultimately a uniquely gullible type of chump, considering the relatively picayune takings he'll enjoy from his success compared to the enormous collective monetary advantages to be afforded his handlers by successful implantation of their scheme.
"The fourth pillar of Project 2025 is our 180-day Transition Playbook and includes a comprehensive, concrete transition plan for each federal agency. Only through the implementation of specific action plans at each agency will the next conservative presidential Administration be successful.
Pillar IV will provide the next President a roadmap for doing just that."
The details are presumably too ugly and controversial to disclose lest they cement the failure of the carrier candidate.
-
Charlie_Brown at 23:59 PM on 7 August 2024CO2 lags temperature
Bob Loblaw @ 670:
That level of water chemistry is not needed to convey or understand the concepts of equilibrium and lead/lag for CO2 and temperature. It would be needed to go on to explain acidification or total dissolved carbon.
-
Bob Loblaw at 10:28 AM on 7 August 2024CO2 lags temperature
Charlie Brown @ 669:
One needs to be careful about referencing Henry's Law when it comes to CO2. CO2 does not just dissolve in water - it ends up dissociating and forming carbonic acid. This complicates the solubility equations.
SkS has a very good series on ocean acidification - in 20 parts. The 9th part discusses Henry's Law. The entire series is summarized in the 19th and 20th posts in the series.































 Arguments
Arguments



























