Recent Comments
Prev 867 868 869 870 871 872 873 874 875 876 877 878 879 880 881 882 Next
Comments 43701 to 43750:
-
Rob Painting at 17:50 PM on 1 September 2013CO2 lags temperature
tcflood - the abstract is here: Abe-Ouchi et al (2013) - Insolation-driven 100,000-year glacial cycles and hysteresis of ice-sheet volume.
Sounds interesting. Note that it relates to the 100,000-year cycle - as stated in the abstract:
"Carbon dioxide is involved, but is not determinative, in the evolution of the 100,000-year glacial cycles."
Your comment omits this crucial bit of information.
-
tcflood at 16:08 PM on 1 September 2013CO2 lags temperature
Sorry. For completeness, the authors on that Nature paper are Ayako Abe-Ouchi, Fuyuki Saito, Kenji Kawamura, Maureen E. Raymo, Jun’ichi Okuno, Kunio Takahashi, and Heinz Blatter.
-
tcflood at 15:58 PM on 1 September 2013CO2 lags temperature
I have always been bothered by the lack of a detailed and convincing explanation for the 100 ky periodicity of the last four ice ages when the periodicity of northern temperate zone insolation intensities from Milankovich cycles (my understanding is that this is the important component of these cycles) is close to 20 ky. A recent paper in Nature (Vol 500, August 8, 2013, page 190) presents an explicit explanation and also describes how CO2 has a role but is not determinative in ice age/interglacial cycles. What do you people at SkS think about this paper?
-
dvaytw at 15:39 PM on 1 September 2013It's El Niño
Thanks for the info, Rob, but what do you think of my characterization of the problem with his hypothesis? I'm trying to sum it up in a way that is hospitable to people like myself without a solid science background.
-
Dave123 at 11:36 AM on 1 September 2013Global warming...still happening
I'm imaging the climastrology reponse to Dan Bailey's repost of the excellent Climate Central graphic-
begin simulation exercise
'Ya see...we had 4 steps up and a plateau from 1900-1940, so we've had 4 steps up now, so we're due for another plateau!!! If only climate scientist could count with the fingers on one hand'
(end simulation exercise).
-
Andy Skuce at 09:57 AM on 1 September 2013The Beginners Guide to Representative Concentration Pathways - Part 3
I tend to agree with chriskoz that the level of CCS envisaged in all but one (RCP8.5) of the pathways seems unrealistically high. Also, the amount of solar/wind/geothermal generation seems very low; this category is the smallest (or nearly so) in all four pathways in Figure 14 above.

This triangular diagram from van Vuuren et al is interesting. In all four pathways, fossil fuels (with or without CCS) make up greater than 50% of energy technologies by 2100, and it is only in the final few decades that the fossil fuel share drops below 62.5% and only in two of the pathways (3PD and 4). Considering that absolute levels of fossil fuel consumption rise in all four cases (Fig 14) and that much of the CCS activity, especially geological storage, will likely be handled by future versions of the big oil companies, the demise of Exxon et al may not be a foregone conclusion.
I know, these are not forecasts, just projections, but it is surprising that none of the pathways models the kind of energy transition (i.e.,renewables-dominant, with little fossil fuel and CCS) that many green-minded people envisage.
-
Jonas at 09:47 AM on 1 September 20132013 SkS Weekly News Roundup #35B
Link for second article is missing. Here it is: http://grist.org/climate-energy/carbon-targets-carbon-taxes-and-the-search-for-archimedes-lever
Moderator Response:[JH] Missing link inserted. Thanks for bringing this to our attention.
-
jonthed at 07:18 AM on 1 September 2013Global warming...still happening
I still don't understand why this argument hasn't already been put to bed. I've seen several news articles and papers in recent months talking about the pause in warming being linked ot the enso cycle, as if this was news? Surely this was patently apparent ever since this chart was first circulated:
http://skepticalscience.com/graphics.php?g=67
All trends continue upward, it's just we're still in the la nina and neutral territory of the spectrum.
It seems obvious to me that the next El Nino is gonna prove decisively what this chart already shows.
-
Joel_Huberman at 05:33 AM on 1 September 2013Global warming...still happening
Thanks, Daniel (#16), for the very nice graph from Climate Central. One from NASA that conveys a similar lesson can be found here. In the NASA graph, small differences can be seen among the four datasets/interpretations. The dataset/interpretation from the Japanese Meteorological Society shows 1998 as the warmest year on record, but the datasets/interpretations from the other three sources show 2005 and 2010 as being slightly warmer. Consistent with the graph from Climate Central, the graph from NASA clearly shows (by simple eyeballing) that, regardless of dataset/interpretation, the decade 2001-2011 was warmer than any preceding decade.
-
Daniel Bailey at 04:38 AM on 1 September 2013Global warming...still happening
Joel, depending on the datasets used, 2005 and/or 2010 each equalled 1998 as the warmest year for the surface temperature record. However, when viewed as decadal averages, the most recent decade has been the warmest, by far:
-
empirical_bayes at 03:05 AM on 1 September 2013Global warming...still happening
@VeryTallGuy for #2, @Ari Jokimäki for #7,
I am not familiar in depth with the Santer et al (2011) calculations, but a quick look at their Appendix and their Equation (A1) and runup to that suggests these a pretty ad hoc. They themselves say, in their paragraph numbered "68":
While non‐independence of samples is an important
issue in formal statistical significance testing, it is not a
serious concern here. This is because our pc(i)′ and pf (i)′
values are not used as a basis for formal statistical tests.
Instead, they simply provide useful information on whether
observed TLT trends are unusually large relative to modelbased
estimates of unforced trends, or unusually small relative
to model estimates of externally‐forced trends.I can't comment on what they are trying to do here, but it's fair to say this is no conventional kind of statistical significance being calculated. Worse, statistical "significance" cannot be properly used in the way VeryTallGuy seeks to do so, and as many do. "Significance" has a specific technical meaning, and I would strongly recommend reviewing that, e.g., at http://en.wikipedia.org/wiki/Statistical_hypothesis_testing#Interpretation See also http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1756718/
The problems classical significance testing or hypothesis testing has in complicated situations is one of many reasons why modern statistical inference is couched in the Bayesian view. See the text, J. Kruschke, Doing Bayesian Data Analysis for a great introduction. Alternatively, check out http://hypergeometric.wordpress.com/2013/08/22/soon-you-will-be-one-of-us/
-
Joel_Huberman at 01:42 AM on 1 September 2013Global warming...still happening
Thanks to both Ari and the moderator (DB) for the explanation. I hadn't realized that the data shown in the Berkeley Earth summary graph had been corrected in the same way that the red line in Ari's graph had been corrected. The explanatory legend at the Berkeley Earth site didn't make that clear. But it makes sense, because the red line in Ari's graph resembles all four lines in the Berkeley Earth summary graph. I conclude that the uncorrected global temperature in 1998 has not yet been exceeded by any subsequent uncorrected annual average global temperature.
-
Ari Jokimäki at 01:24 AM on 1 September 2013Global warming...still happening
Joel, the caption of the figure in question mentions the data-source for the pink line: "one year running mean averaged from all surface temperature analyses". Further details are available in Rahmstorf et al. paper to which there's a link in the references section.
-
Joel_Huberman at 00:57 AM on 1 September 2013Global warming...still happening
Very nice article, but there's one thing I don't understand. The pink line in the second figure suggests that the uncorrected global temperature in 1998 was higher than for any succeeding year. In contrast, other global temperature measurements, such as the four measurements graphed on the "Berkeley Earth" summary page suggest temperatures higher than 1998 in several of the succeeding years. My guess is that a different global temperature data set was used to generate the pink line, different from the data sets used to create the lines in the Berkeley Earth summary graph (NASA GISS, NOAA/NCDC, Hadley/CRU, and Berkeley Earth). Am I right? In any case, it would always be a good idea to provide the source of any data set used to make graphs.
Moderator Response:[DB] The paragraph immediately following the legend for that graphic fully explains the corrections done:
As we can see from the graph, surface temperature changes shown in pink sometimes go outside the projections. This is because some factors are not included in IPCC projections. Such factors are solar activity changes and eruptions of volcanoes. Additionally, the variation of El Niño/La Niña is random and therefore it doesn't change in simulations at the same time it does in real life. IPCC projections are combined results from many simulations, so the El Niño/La Niña variations of different simulations tend to cancel out when simulation results are combined. This means that the projections don't actually include El Niño/La Niña variation either. It should be noted that even if surface temperature shown in pink doesn't stay within limits of projections, it does stay within limits of all individual simulations (not shown in the Figure above).
Once these exogenous and transitory factors are removed, the underlying trend emerges, shown in red.
The BEST version, using a similar accounting:
-
BillEverett at 23:58 PM on 31 August 20132013 SkS Weekly News Roundup #35B
Several things in the livescience article "Cooler Pacific Ocean May Explain Climate Change Paradox" disturbed me.
1. The Pacific Ocean (as a whole ocean) is warmer, not cooler. The equatorial surface has been cooler, which is what happens when we have a turnover of warmed surface water going down and cold deep water coming up. "Cooler Pacific Surface" would be more accurate.
2. What "Climate Change Paradox"? Deniers claiming "global warming has stopped" doesn't create any climate change paradox, let alone a paradox that needs to be explained.
3. The picture caption "A thermometer in the Earth shows increasing global climate sensitivity" is absurd.
The actual article isn't so bad, although I wish that a little more clarity had distinguished the difference between the surface and the ocean, making it clear that we can have a cool-warm surface alternation with a cool or cooling ocean or with a warm or warming ocean, and that we currently have the surface alternation with a seriously warming ocean (bad news). The packaging of the story (not likely the fault of the writer) shows a level of scientific understanding that I would expect to see on a denier site.
-
Dave123 at 21:52 PM on 31 August 2013Global warming...still happening
Solomon isn't seeing any recent increase in stratospheric water vapor, and Chapman's contribution spectrogram seems to confirm this. Over a longer period then we need to look to Dessler (2010) for long term water vapor feedbacks. You can just bet that some of the denierrati will jump on this to argue that there are no positive feedbacks, that we're limited to forcing from increased CO2 only, ECS is about 1C and thus all is right with the world.
-
Rob Painting at 19:17 PM on 31 August 2013It's El Niño
Actually SkS has been writing about this oscillation for years, a.k.a the Interdecadal Pacific Oscillation (IPO). For the latest discussion see this recent(ish) SkS post: A Looming Climate Shift: Will Ocean Heat Come Back to Haunt us?.
The oceans are warming due to the increased (enhanced) Greenhouse Effect (this is the upward slope in the 2nd graphic below), but the wind-driven ocean circulation moves back-and-forth between intense and sluggish phases, which results in the 'hiatus' and 'accelerated warming' decades. The net effect is illustrated in the graphics below:


SkS will have upcoming posts/rebuttals explaining this in some more detail. You'll see how the observations by Kosaka & Xie (2013) tie in nicely with the wind-driven ocean circulation.
Good to see Bob is slowly coming around to our way of thinking though. He still has a looooong way to go.
-
dvaytw at 18:46 PM on 31 August 2013It's El Niño
Hey guys: Bob Tisdale’s crowing about the recent studies attributing slowed surface warming to La Nina:
“Anyone with a little common sense who’s reading the abstract and the hype around the blogosphere and the Meehl et al papers will logically now be asking: if La Niña events can stop global warming, then how much do El Niño events contribute? 50%? The climate science community is actually hurting itself when they fail to answer the obvious questions.
And what about the Atlantic Multidecadal Oscillation (AMO)? What happens to global surface temperatures when the AMO also peaks and no longer contributes to the warming?
The climate science community skirts the common-sense questions, so no one takes them seriously."
http://wattsupwiththat.com/2013/08/28/another-paper-blames-enso-for-the-warming-hiatus/#more-92630
How’s this for a summary argument against the Tisdalian hypothesis (if it constitutes one):
Tisdale and his crowd at WUWT seem to think temperature is just a number that just moves up and down somewhat arbitrarily, like a stock price. They don't conceptualize things properly in terms of heat energy, which can't be created or destroyed.
ENSO doesn’t generate, absorb or destroy heat. So when Tisdale says La Nina “stops” global warming and El Nino “contributes”, he’s got it totally wrong. Nothing is being stopped – the heat energy is simply being moved around. That's why we use the term "oscillation".And the proper question to ask Tisdale is simply, Where is the heat coming from?
I'm probably getting it all mucked up, as I have little science background, but I'm trying to put it in easy terms for non-sciencish people like myself.
Also, what do you think of this graphic from Hotwhopper:
ENSO without AGW:
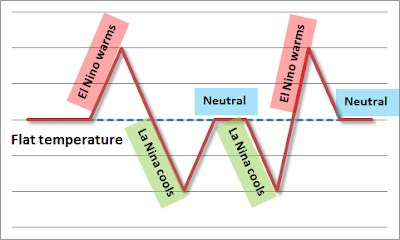
ENSO without AGW:
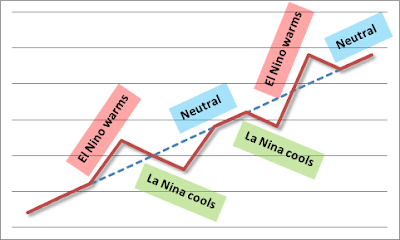
-
CBlargh at 15:54 PM on 31 August 2013CO2 was higher in the late Ordovician
@Tom:
Wait! They may not be unrelated! I just read a paper suggesting roots increase weathering.
<a href = "http://www.pnas.org/content/98/8/4290.full.pdf">Falkowski and Rosenthal</a>
If the late Ordovician was the first time land rhizomes evolved, it would suggest to me it could have caused a pulse of calcium which pulled down atmospheric carbon in the sea, not necessarily in freshwater bogs through the land plants themselves. -
Daniel Bailey at 12:06 PM on 31 August 2013Greenhouse Effect Basics: Warm Earth, Cold Atmosphere
Molecular visualizations of CO2 from the GIF's (from Timothy Chase's website):
Ground State Mode
Pure Symmetric Stretching Mode
The pure symmetric stretching mode v1 of CO2. While this is a mode that may gain and lose energy collisionally it is not infrared (IR) active as there is no transient electric dipole.
Bending Mode V2
The bending mode v2 of CO2, responsible for the 15.00 μm (wavenumber 667 cm-1) band -- the mode dominating the enhanced greenhouse effect and that primarily used by AIRS. This is infrared (IR) active due to a transient dipole: bending results in charge being asymmetrically distributed with net positive near the carbon atom and negative near the two oxygen atoms.
And
Asymmetic Stretching Mode V3
The asymmetric stretching mode v3 of CO2 is responsible for the 4.26 μm (wavenumber of 2349 cm-1) band. The asymmetic stretch result in a net positive charge near the carbon atom and a net negative charge with the isolated oxygen atom, creating an electric dipole and making it infrared (IR) active. Given the range of atmospheric temperatures and concentrations of CO2 the bending mode v2 plays a greater role in climate change.
-
Daniel Bailey at 11:50 AM on 31 August 2013Greenhouse Effect Basics: Warm Earth, Cold Atmosphere
It might be helpful to visualize the various vibrational modes of CO2 with these animated GIFs:
-
Phil at 07:18 AM on 31 August 2013Greenhouse Effect Basics: Warm Earth, Cold Atmosphere
MThompson @81. I think you are both right and wrong. The asymmetric stretch is stronger than the bend, however the important fact you are missing is the distribution of IR radiation emitted by planet Earth. This is a (near) black body distribution, and the peak (at 288K) almost co-incides with the CO2 bend. Thus the bend plays a more important role simply because there is more radiation in the Earths emission spectrum to absorb.
-
MThompson at 05:19 AM on 31 August 2013Greenhouse Effect Basics: Warm Earth, Cold Atmosphere
Many thanks to Phil and KR for educating me on this topic. Naturally I have done considerable reading online, but many times the explanations are not clear to me because of the misapplication of concepts and poor analogies.
From my reading it seems that asymmetric stretch is the primary vibrational mode for CO2 and is some 13x more intense than the two bending modes combined. So in my understanding from this discussion and other reading is that CO2 is capturing the blackbody radiation of the earth at these wavelengths. Additionally there is some broadening of the lines that allows more than just the two primary “peaks” to be absorbed, and that broadening increases the total energy stored in vibrational modes of C02.
Please let me know if I have a good mental image of the process. -
Yvan Dutil at 05:03 AM on 31 August 2013Global warming...still happening
For me, it is pretty obvious that the graph of Chapman et al (2013) will become a classic to illustrate the greenhouse gaz effect.
-
NASA GISS adjustments introduce warming bias
Apparently so. I've commented on the subject here.
-
Watts' New Paper - Analysis and Critique
Continuing from an unfinished page here:
As I pointed out on a WUWT thread that claimed adjustments were distorting the data,
It could be argued that it’s better to look at raw temperature data than data with these various adjustments for known biases. It could also be argued that it’s worth not cleaning the dust and oil off the lenses of your telescope when looking at the stars. I consider these statements roughly equivalent, and (IMO) would have to disagree.
Various replies (arguments from authority, etc) followed, mostly ignoring the rather copious literature on time of observation bias.
-
BaerbelW at 03:10 AM on 31 August 2013Where SkS-Material gets used - Coursera's Climate Literacy Course
Richard Alley's course I mentioned above has been postponed until January 6, 2014:
Energy, the Environment and Our Future - Penn. State University
"Get Rich and Save the Earth…Or Else! Learn about the past, present, and possible futures of human energy use."This suits me just fine as I won't have to juggle two courses running in parallel (I'm currently enrolled in University of Melbourne's "Climate Change")!
-
dvaytw at 02:47 AM on 31 August 2013NASA GISS adjustments introduce warming bias
I'm assuming this page is under construction? I'm asking because I've just been hit with this line in a debate.
-
Global warming...still happening
Tamino gave an even better summary later in the thread:
If you flip a coin 10 times and all 10 flips give "heads" then you've got strong evidence the coin is biased. If you flip a coin 10 times and record the result, then repeat that process 1000 times, and one of the "flip sets" is 10 heads, you do NOT have evidence of a biased coin. If you then report the single set of 10 which gave 10 heads, without mentioning the 999 sets of 10 which didn't, you're dishonest (maybe to yourself). Does that make it clear?
-
Global warming...still happening
Regarding Santer et al and time required for trend significance, Tamino posted something very interesting a few years ago:
Cherry Snow - It turns out that if you cherry-pick an extrema starting point for your trend identification (as opposed to the more frequent values near the trend), the t-value needed to establish trend significance almost doubles.
In other words, if you select a trend point such as the 1998 El Nino as your start, as done over and over by 'skeptics', it distorts the statistics such that the trend over that period is less significant, so that what would be reasonable (say, 17 years) is insufficient due to that cherry-pick.
Cherry-picking extrema end points weakens trend significance, making the oft-repeated "16 year" claims even less meaningful - they have the least trend significance of any possible data selection of that length.
-
Ari Jokimäki at 00:12 AM on 31 August 2013Global warming...still happening
VeryTallGuy #2: I think your interpretation of Santer et al. makes sense, but I'm not a statistics expert.
Chriskoz #5: Sorry, the vertical axis is indeed insufficiently labeled (but it's like that already in the Chapman et al. paper). The vertical axis shows Brightness Temperature.
I don't think I said anything about total outgoing IR, but only the "characteristic absorption frequencies of greenhouse gases". The study of Chapman et al. shows that the IR absorption of said greenhouse gases has increased over the ten year study period.
-
One Planet Only Forever at 23:51 PM on 30 August 2013Global warming...still happening
The indepth presentation is interesting and infromative. However, I prefer a simpler statement.
The warming since 1998 can best be evaluated after the next signifcant El Nino of the magnitude that occurred in 1998 occurs. There are other variable factors influencing the surface temperature, but the global average surface temperature history shows very strong "bumps" in the global averages when significant El Ninos are acting.
So the implication that a specific minimum number of years of data is required to establish statistical significance is not correct. The major variable influencing factors need to recur in the data being evaluated, whatever time that takes.
-
mitch at 21:43 PM on 30 August 2013Abraham et al. (2013) explore the known unknowns in the oceans and global warming
Ranyl in (1): The reason that researchers don't make these available in open access journals is because that there is a cost of about 2000 dollars to make them open access. Someone has to pay for the residual cost (after volunteer reviews, etc.) of editing, formatting, posting, and maintaining the journal archive.
If I charge the 2000 dollars to a grant, then I must either cut back the pay to myself or my students, or I must cut back the number of analyses that are done. There is no free lunch.
-
chriskoz at 21:31 PM on 30 August 2013Global warming...still happening
Ari,
Last figure is unreadable, especially the axis legends.
At least I can imagine on horizontal axis the values of spectral numbers where known CO2 and H2O absorption bands are. But I have no clue what is on vertical axis.
And how do you read from said figure that total outgoing IR in the spectrum shown has decreased over last decade as implied by the caption?
-
TonyW at 20:17 PM on 30 August 2013The Beginners Guide to Representative Concentration Pathways - Part 3
Sorry, I meant to add that it's really not so much reserves that matter but the likely rate of production, as fossil fuels get tougher and tougher to extract (e.g. oil sands might be an enormous reserve but they won't be produced at anything like the rate of conventional oil from similar sized reserves). -
TonyW at 20:14 PM on 30 August 2013The Beginners Guide to Representative Concentration Pathways - Part 3
It's not clear to me that any consideration has been taken of energy reserves in these pathways. For example, oil use in RCP8.5 is shown rising to double what it is today? That seems unreasonable, given that even though a peak of all liquids has not been experienced, the energy content of what is produced is not substantially different from 8 years ago. It may not be possible to increase the energy from oil much in the future. If this is true, then any pathway that projects much increase would be unusable. The same applies for coal use, given that the data on coal reserves is poor but some analyses I've seen suggest energy produced from coal could well peak before mid-century, even within a decade or two. Ditto natural gas, despite the hype of fracking (US dry gas production has stalled for the last 18 months, for example).Do the RCPs take account of plausible reserve scenarios at all? I think they need to -
Lanfear at 19:25 PM on 30 August 2013Abraham et al. (2013) explore the known unknowns in the oceans and global warming
The paper in question seems to be available here.
Many a time you can google these by putting the title of the paper in quotes, and then adding the filetype:pdf definition.
-
VeryTallGuy at 19:20 PM on 30 August 2013Global warming...still happening
Let's then look at the trends recently.
I used GISS, and by my reckoning, there are *no* years in the past 33 where the trend is negative.
This applies whether the trend is defined as end – to – end, or least squares best fit.
Going for the least squares best fit approach, which seems more reliable, the last time there was a 17 year negative trend was 1976, and there are only 3 in the past 50 years (’67, ’68, ’76)
On that basis, there is nothing unusual whatever about the current “pause”, indeed it is expected and could even be argued as overdue.
-
Ari Jokimäki at 19:14 PM on 30 August 2013Global warming...still happening
Thanks, Shoyemore. I think that there might be a separate article on that paper in SkS in near future.
-
VeryTallGuy at 19:13 PM on 30 August 2013Global warming...still happening
I think the meaning of statistical significance has been misapplied to the current hiatus/pause.
As I understand Santer (please correct me if I'm wrong) defines statistical significance as a 95% probability of a positive trend over 17 years. As applied to a "pause" in surface temperatures during a period of rising Co2, let's define that as
"During a period when CO2 rises at 1.5ppm per year, we expect, for any given year, a 5% probability that the trend across the preceeding 17 years will display a zero or negative value"
Let's define modern warming as being over the past 50 years (during which time Co2 has risen at ca 1.5ppm/year)
We then *expect* one in twenty of the past 33 years (those where we can measure the previous 17) to show a negative or zero trend for the preceeding 17.
Our prediction is then that roughly one or two years of the past 33 will have a negative trend.
Feedback on if this is a correct interpretation of the science would be much appreciated.
-
shoyemore at 17:31 PM on 30 August 2013Global warming...still happening
Ari,
This paper is causing some current stir ... it may be one you would like to comment on in the light of this excellent review of papers & perhaps add to your list.
Recent global-warming hiatus tied to equatorial Pacific surface cooling
Thanks.
-
Tom Curtis at 14:14 PM on 30 August 2013Human CO2 is a tiny % of CO2 emissions
YubeDude @252, I am not aware of any use of O18 as a marker of CO2 of organic origin. Of course, I am not expert in the field, so that only means it has not made it into popular presentations (or the IPCC reports). Further, searching google scholar does not readilly turn up studies using O18 as a marker for the organic origin of CO2.
I suspect it is possible in principle. However, fractionation of O18 in respiration is a confounding factor, and likely to be stronger. Further, atmospheric O18/O16 ratios will vary more with time than than C13/C12 ratios due to the fractionation of O18 in water by evaporation and condensation. No doubt there are other complexities of which I am not aware as well.
Sorry I can't help anymore.
-
YubeDude at 13:18 PM on 30 August 2013Human CO2 is a tiny % of CO2 emissions
TY Tom for the reply. I understand about the carbon ratio and the meaning what I am interested in is if CO2 also caries the O-18 marker that would link that particular molecule of CO2 directly to combustion and not just a sink release or another anthropogenic use of older carbon sources such as chemical manufacturing that is petro-chemically based.
I am wondering if O-18 has any merit whitin the topic as I see no mention or research that attempts to link or associate its presence with A-CO2.
No quibble on my part as to the evidence rather just a currisoity in regards to this darn O-18. Thanks in advace for any light you may be able to shed.
-
jdixon1980 at 04:21 AM on 30 August 2013Abraham et al. (2013) explore the known unknowns in the oceans and global warming
ranyl @1 Sometimes they are free, like the Trenberth paper referenced at the end (Balmaseda et al.).
The journals probably can't afford to make every important paper free. But the amazing thing is the wealth of knowledge that you can get right here for free, thanks to so many highly qualified people who, as I understand it, donate their time in writing these posts, and who also frequently engage questions and criticisms down here in the comments section.
-
jja at 02:25 AM on 30 August 2013How much will sea levels rise in the 21st Century?
in the link you posted the authors cite the A2r scenario, updated with current developing country projections: "
"The RCP8.5 is based on the A2r scenario (Riahi et al. 2007), which provides an updated and revised quantification of the original IPCC A2 SRES scenario storyline (Nakicenovic et al. 2000). With a few exceptions, including an updated base year calibration (to 2005) and a revised representation of short-term energy trends, especially in developing countries, the RCP8.5 builds thus upon the socio-economic and demographic background, resource assumptions and technological base of the A2r scenario."
if you look at the final values of CH4 and CO2 atmospheric concentrations in 2100 you will see that the A2 scenario is approximately 15% below the RCP 8.5 high error margin estimation. You will also find that the A1Fl concentrations match that value much more closely.
2. This is the limitation of science, in a non-linear environment. PIOMAS has been verified by Cryosat-2. This has not been utilized in the AR5. The non-linear response of multiple coupled systems to the loss of arctic sea ice will produce traumatic shifts that are unforetolled by the AR5. The paper I quoted is published in 2008, this is not a "new" paper.
3. Your estimates of climate sensitivity are based primarily on paleoclimate data. However, you do not have a statistically signficant number of sample points for the current climate regime. So, you throw out the anomalous data that indicates that there is a much higher climate sensitivity during interglacials. You justify this because you do not understand why a runaway warming did not occur if sensitivities were actually that much higher during the interglacials.
In addition, the issues that I have brought up are not uncertainties, they are unknowns. The difference is that the unknowns are not contained in the current models. The body of evidence indicates that these unknowns (defined as proesses with impacts that are determined to be real, potentially catastrophic, but currently undefined and so not included in the model) will have effect, and that the potential effects of their cumulative impacts will surpass even the worst case scenarios.
The 3,425 ZJ cumulative energy deposition is your value not mine. I assume a current TOA imbalance of .75 W/m^2 and a 2100 TOA imbalance value of 3.5 W/m^2 (linear and proportional to RCP 8.5). Yeilding an average TOA imbalance of 2.125 W/m^2 between now and 2100, producing the cumulative energy deposition of 2,939 ZJ. I used RCP 8.5 for continuity of discussion, I do believe that RCP 10.0 is more likely given the albedo and carbon cycle feedbacks (as well as emission scenarios) posited in @16.
6. The .44 TOA came from your post@ 43 when you said, "That formula predicts a current TOA energy imbalance of 0.44 W/m^2, and a 2100 imbalance of 1.76"
you did not show your math so I reproduce it here:
1. TOA imbalace will be have constant proportionality to RF between now and 2100 (you say this is conservative)
2. Current TOA is .63, RF is 1.635(to pre-industrial) Ratio is 35.8/100
3. you then used RCP 6.5 for your calculation, I used RCP 8.5
4. 8.5 * 35.8/100 = 3.27 W/m^2
5. If I use a starting TOA of .75 then my values are slightly higher for TOA in 2100 = ~3.57. The rate of warming from the last interglacial is approximately 200X slower than current warming rates. This is an undeniable truth. The ice will necessarily melt more slowly. The Milankovich cycles are not GHG driven events. I am sure you know this.
I would ask that future discussions by AR5 reviewers list clearly and succinctly whether or not the following unknowns are contained within their models.
1. The cessation of Anthropogenic SOx emissions in 2070
2. The reduction of the OTC by 80% in 2060 and its effects on North Atlantic carbon sink.
3. The effect of drought on the Amazonian basin by year 2050 on natural carbon sequestration
4. The potential for boreal peat carbon emissions in a +4C environment
5. The step-change in annual surface temperatures produced in the arctic for an ice-free state in the summer.
6. The total emission potential from the ESAS under a regime of consistent +10C water temperatures in late summer.
7. The localized ocean warming effect on the West Antarctic Ice Shelf under a regime of 80% reduction of the OTC.
8. The projections of Western United states drought under a regime of a summer ice-free arctic in 2030.
9. Accelerated Permafrost emissions due to an ice free summer state in 2030.
10. The effects of these non-analyzed carbon cycle and albedo feedback scenarios within an environment where Carbon Capture and Sequestration is NOT utilized by developing countries and ECS for 2XCO2 is actually 4.5C.The above would be a very useful study. It would also provide a reasonable basis for the collective mea culpa due to the human race (and the Nobel Committee) when the arctic ice DOES disappear in 2030 and catastrophic warming events begin to reveal these unknowns to be real and disasterous.
-
joeygoze9259 at 01:25 AM on 30 August 2013Abraham et al. (2013) explore the known unknowns in the oceans and global warming
In abstract, states that sea level rise is at 3 mm/yr. This sounds low compared to other estimates I have read. see http://www.nap.edu/openbook.php?record_id=12782&page=244
-
jdixon1980 at 01:12 AM on 30 August 2013CO2 limits will hurt the poor
A dissenter/denier/contrarian/whatever friend of mine keeps bringing up the issue of forgoing fossil-fuel based industrialization being a lost opportunity to alleviate the world's worst poverty. (We generally have these discussions on my Facebook timeline.) He also brings up the whole gamut of meritless "scientific" arguments (solar activity, Milankovitch cycles, cosmic rays, nameless "natural cycles," platitudes about "uncertainty/complexity") as well as the usual conspiracy theories about AGW theory being a commie plot to bring down the west, etc., all of which made it hard for me to take his poverty argument seriously for a long time.
I'm not even sure that alleviation of poverty argument is a good argument against aggressive emissions reductions. It's just that I haven't been able to find any satisfying discussion of it on the web. This may be because the kind of analysis I would ideally like to see would probably take a lot of experts a long time to put together: I would like to see analysis of an emissions pathway that at least a majority of scientists would consider prudent (i.e., at a bare minimum avoiding any significant risk of a "Hell on Earth" scenario within the next X number of years - 100? 150?, such as requiring mass evacuations of coastal cities and low-lying island nations, etc.) in terms of what increase in power generation per capita can feasibly be distributed to the poorest regions of the world within the constraints of that emissions pathway by X date, what the difference is (if any) between that increase and the increase that would be feasible by X date in a free-for-all scenario with no emissions restrictions, and what that difference implies in terms of a sacrifice in quality of life (if any) that today's poor will have to make for the sake of avoiding climate catastrophe. I have no idea what that kind of analysis would entail, or whether it might take so long or depend on so many political unknowns (for example) that it wouldn't be worth doing, but I would like to see somebody take a stab at it.
Of course, there is another elephant in the room, which is that if our rate of consumption of global resources across the board already far exceeds Earth's ability to replenish them (see "Earth Overshoot Day," "Ecological Debt Day," which the Global Footprint Network says that we hit this year on August 20), then it's frightening to think what would happen if every country in the world had a population that consumed like America's or Europe's populations. This doesn't change the fact that some people live on appallingly little and consume far less than their fair share of resources, but it would be crazy to pursue policies aimed toward making sure that everybody in the world overconsumes to the same degree as I do in America. They have a right to scale up consumption, but not to our current level, and I have no right to remain at my current level of consumption. Reducing inequality is a noble goal, but if the Global Footprint Network is anywhere close to right, then reducing inequality will have to involve meeting somewhere in the middle or we are screwed.
-
Tom Curtis at 21:18 PM on 29 August 2013How much will sea levels rise in the 21st Century?
jja @50:
1) RCP 8.5 is based on the IPCC AR4 A2 scenario (see discussion at the top of page 34).
2) Treating every newly published result that suggests worse outcomes than the consensus as gospel is not science, it is advocacy. Publication in only the first step, and until a result has had a chance to be reviewed by the scientific community at large, and responses published, or the results accepted and cited, they should be regarded as provisional at best. That you are not only seizing on such results but ignoring (equally provisional) results that point in the other direction shows very clearly that it is not the science driving your view point.
3) It is true that the ice and snow albedo feedbacks are larger than expected in the models. The models, however, include many feedbacks with significant uncertainties - and just be chance we would expect some to be under-estimated and some over-estimated. Pointing to one that is under-estimated and concluding from that that the climate sensitivity is under-estimated without out a full survey of the evidence with regard to all feedbacks is, again, not science but advocacy. Afterall, from the nature of uncertainty we expect there to be some under-estimates, and finding one tells us nothing about the overall balance.
4) Measurements of climate sensitivity from paleodata by their nature include all feedbacks. Therefore, the evidence from that source is unaffected by findings regarding the strength of particular feedbacks. If we find one feedback to be stronger, then of necessity the balance of remaining feedbacks must be weaker because the total effect of all feedbacks are already included in the estimate. As an ECS of 3 +/- 1.5 C per doubling of CO2 is a robust result from paleoclimate analysis of ECS, finding an enhanced ice and snow albedo feedback does not make an ECS greater than 4.5 C per doubling of CO2 appreciably more likely.
Given points (3) and (4), I think there can be no doubt that your scenario is a "worst case" scenario, and is not supported by the current balance of scientific evidence (no matter how well supported by a cherry picked sample of that evidence).
5) Contrary to your supposition, the 2110 ZJ (RCP 8.5) result "assumes" a TOA energy imbalance of 0.686 W/m^2 in 2014, and is consonant with Trenberth's result (although notice again your selection of the largest available value as being bedrock). The 3425 ZJ (RCP 10) result "assumes" a TOA energy imbalance of 1.115 W/m^2 for 2014. Between them, therefore, they bracket the Trenberth result, and by your logic the RCP 10 value is an overestimate. Therefore, take that value and you still need to propose a mechanism to raft a volume of ice equal to 44% of the volume of Greenland's Ice Sheet. You refuse to confront this absolute necessity in your scenario, and until you do you don't have a theory worth considering.
6) I note in passing that your maths regarding energy balances is all over the place, and way off. Thus, in your comment @47 you claim my linear estimate based on RCP 8.5 and ECS2xCO2 as assuming a current TOA energy imbalance of 0.44 W/m^2 (it actually assumes 0.6 W/m^2). You further claim that using an current imbalance of 0.75 W/m^2 would result in a mean annual imbalance of 3.65 W/m^2. The correct value is 1.525 W/m^2.
Finally,
7) Your explanation of why ice sheets melted slower coming out of the last glacial amounts to asserting that ice sheets melted slower because ice sheets melted slower (ie, the albedo reduced slower). The reasoning is circular and provides no support for your hypothesis, and gives us no reason to ignore the lessons of the past.
-
chriskoz at 21:00 PM on 29 August 2013The Beginners Guide to Representative Concentration Pathways - Part 3
In find the belief in CCS by (van Vuuren et.al. 2011) surprising. Especially if you look at Figure 14: RCP2.6 in 2100 burns twice as much coal as has been in 2000. Is it realy realistic that so much coal be burned and yet the CO2 will decline from a peak of about 450ppm in mid century to current 400ppm level? That's contradictory or relying on CCS (technology not yet proven) doing this miracle. That's like the reliance on future generations to perfect that technology to 100% efficiency or to start scrubbing CO2 from the air.
I would rather expect that the renewable mixture (hydro/PV/thermal/wind/etc) to take a large chunk of energy in RCP2.6, basically replacing that big chunk of coal. Said renewables, boosted with gas to balance their intermittent nature, are proven, existing technology. But they actually take the smallest chunk in future energy mixture among all scenarios on Figure 14. How could it be, given RCP2.6 is the most agressive mitigation scenario? Does it mean that the authors "do not believe" in this technology as opposed on their reliance on CCS?
I think that either RCP2.6 on Figure 14 is erroneous, or my knowledge about CCS is incomplete: i.e. CCS is proven to be possible on 100% scale and as cheap as to successfuly outcompete the renewables by 2100. Can someone convince me to the latter?
-
ranyl at 18:17 PM on 29 August 2013Abraham et al. (2013) explore the known unknowns in the oceans and global warming
Shame the paper is behind a paywall.
Would have been nice to able to read such an important topic.
Why don't researchers with such important works make them available in name of open access and proper knowledge distribution at this critical time?
Prev 867 868 869 870 871 872 873 874 875 876 877 878 879 880 881 882 Next































 Arguments
Arguments


































