OA not OK part 6: Always take the weathering
Posted on 16 July 2011 by Doug Mackie
This post is number 6 in a series about ocean acidification. Other posts: Introduction , 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
Weathering refers to the wearing away of rocks by, err well, weather (or birds ).
Carbonate rocks can be CaCO3 (calcium carbonate or limestone) or MgCO3 (magnesium carbonate) or mixed calcium-magnesium carbonate CaMg(CO3)2 (dolomite) and several other less common forms. We will discuss only calcium carbonate, but as calcium and magnesium are members of the same group (i.e. vertical column) on the periodic table, you would just replace Ca with Mg in the equations.
Limestone weathering occurs when rainwater reacts with the carbonate rocks. Rainwater does not have a neutral pH, but this has nothing to do with industrial pollution. Rainwater is in equilibrium with atmospheric CO2, so carbonic acid is formed via equation 7 from post 5. This leads to mildly acidic rainwater with a pH of about 5.7.

(Note: acid rain refers to rain with a pH less than 5.7 due to equilibration of rain with sulphate and nitrate - forming sulphuric and nitric acids - from the burning of fossil fuels and biomass.)
What happens when rain washes over limestone on its way to rivers? In post 1 we introduced Equation 4, the reverse of calcification, which describes the weathering of carbonate rocks:
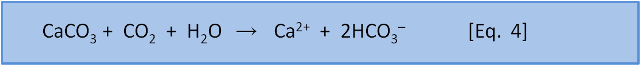
In post 1 we also said that Equation 1, calcification, was a source of CO2. So it should come as no surprise to say that the weathering of carbonate rocks consumes atmospheric CO2.

The result of weathering is that river water contains a lot of carbon species, in the form of bicarbonate and carbonate, compared to seawater.
River water also contains salts. Stephen Jay Gould wrote an engaging essay (republished in the book Eight Little Piggies) about Edmund Halley (he of the comet). Halley, like Gould, was a polymath and one of his ideas was to work out the age of the Earth from the amount of salts in the sea. It isn't clear if Halley knew about the other salts in the sea or if he just meant NaCl, but it doesn't matter as Halley's argument works just as well for carbonate species. Halley began with the observations that:
1) The oceans are not saturated with salts (NaCl and/or carbonate species etc). That is, you can add additional salt to seawater and it will dissolve.
2) River water contains salts.
Halley knew the water was recycled and he knew the salts came from the rivers because of weathering. Halley assumed the salt content must be increasing with time as the rivers washed more salts in, but none ever left. So Halley concluded that if you measured the saltiness (salinity) at the time (1700-ish for Halley) and again in a few hundred years then Halley expected that the salinity would have measurably increased and allow a maximum the age of the Earth to be calculated.
For example, current salinity is about 35 grams per kg of seawater. If it had been 34 g per kg of seawater 300 years ago, the salinity would have increased 1 g/kg over 300 years. Therefore, it would have taken 10,500 years to build up the current 35 g/kg - if we started from salt-free water. The maximum age part is because if the seas had started salty then the Earth would be younger than it seemed. Halley, a young-Earth creationist, expected an answer in the low thousands of years, but was wrong for several reasons.
Halley’s biggest mistake was his assumption that it was a one way process, that salts never leave the ocean. But salts do leave the ocean and are part of a long slow cycle. Halley should have thought this one through as salt deposits (from ancient seas) have been mined by humans for thousands of years. Similarly, calcium carbonate (another salt delivered to the oceans by rivers) also leaves the ocean – as part of the carbon cycle. The most striking example of calcium carbonate leaving the ocean are the White Cliffs of Dover, which are calcium carbonate skeletons of coccolithophores.
We are so familiar with the idea of a carbon cycle now that we don't recall how astonishing the idea would have been only a few hundred years ago that the weathering of carbon from rocks could possibly be part of such a cycle. But, as we shall see in later posts, when atmospheric CO2 is high then more acidic rain causes more weathering and that consumes CO2 to lower the atmospheric CO2. Only problem is that this happens over a geological time scale.
If we do the same sort of calculation that Halley did for salt with bicarbonate HCO3– in rivers then we can get a good estimate of the amount of weathering that happens and thus how much bicarbonate added to the ocean by rivers. Now, we know from Eq. 4 that weathering consumes CO2. So the amount of bicarbonate added to the ocean is equal to the amount of CO2 consumed.
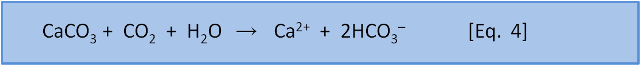
But remember that this CO2 comes from the atmosphere. So it follows that given enough time then that the consumption of CO2 by weathering will remove all the CO2 from the atmosphere. It turns out that amount of weathering is sufficient to remove all CO2 from the atmosphere in 3500 years. Plainly this hasn't happened in the past. Something is returning CO2 to the atmosphere. That something is our Eq. 1 for calcification:
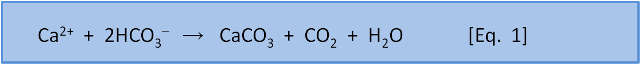
Equation 1 explains the formation of CaCO3 in the ocean from Ca2+ and 2HCO3– derived from weathering. When this CaCO3 dissolves, by Eq. 4, it exactly reverses the original Ca2+ and 2HCO3– derived from weathering that was lost from the ocean by CaCO3 formation, and also consumes the CO2 formed at the same time. However, if CaCO3 is buried in sediments, that Ca2+ and 2HCO3– must now be replaced by further weathering of terrestrial limestone. Fortunately, the CO2 needed to do that has been returned to the atmosphere after CaCO3 formation. Thus closure of the cycle!
In the next post we consider the first stage of what happens when the equilibrium of the oceans is disturbed.
Written by Doug Mackie, Christina McGraw , and Keith Hunter . This post is number 6 in a series about ocean acidification. Other posts: Introduction , 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.































 Arguments
Arguments

























 0
0  0
0 second summary post
second summary post







Comments