Is the CO2 effect saturated?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
The notion that the CO2 effect is 'saturated' is based on a misunderstanding of how the greenhouse effect works. |
|||||||
Climate Myth...
CO2 effect is saturated
"Each unit of CO2 you put into the atmosphere has less and less of a warming impact. Once the atmosphere reaches a saturation point, additional input of CO2 will not really have any major impact. It's like putting insulation in your attic. They give a recommended amount and after that you can stack the insulation up to the roof and it's going to have no impact." (Marc Morano, as quoted by Steve Eliot)
At-a-Glance
This myth relies on the use (or in fact misuse) of a particular word – 'saturated'. When someone comes in from a prolonged downpour, they may well exclaim that they are saturated. They cannot imagine being any wetter. That's casual usage, though.
In science, 'saturated' is a strictly-defined term. For example, in a saturated salt solution, no more salt will dissolve, period. But what's that got to do with heat transfer in Earth's atmosphere? Let's take a look.
Heat-trapping by CO2 in the atmosphere happens because it has the ability to absorb and pass on infra-red radiation – it is a 'greenhouse gas'. Infra-red is just one part of the electromagnetic spectrum, divided by physicists into a series of bands. From the low-frequency end of the spectrum upwards, the bands are as follows: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Gamma rays thus have a very high-frequency. They are the highest-energy form of radiation.
As our understanding of the electromagnetic spectrum developed, it was realised that the radiation consists of particles called 'photons', travelling in waves. The term was coined in 1926 by the celebrated physicist Gilbert Lewis (1875-1946). A photon's energy is related to its wavelength. The shorter the wavelength, the higher the energy, so that the very high-energy gamma-rays have the shortest wavelength of the lot.
Sunshine consists mostly of ultraviolet, visible light and infra-red photons. Objects warmed by the sun then re-emit energy photons at infra-red wavelengths. Like other greenhouse gases, CO2 has the ability to absorb infra-red photons. But CO2 is unlike a mop, which has to be wrung out regularly in order for it to continue working. CO2 molecules do not get filled up with infra-red photons. Not only do they emit their own infra-red photons, but also they are constantly colliding with neighbouring molecules in the air. The constant collisions are important. Every time they happen, energy is shared out between the colliding molecules.
Through those emissions and collisions, CO2 molecules constantly warm their surroundings. This goes on all the time and at all levels in the atmosphere. You cannot say, “CO2 is saturated because the surface-emitted IR is rapidly absorbed”, because you need to take into account the whole atmosphere and its constant, ongoing energy-exchange processes. That means taking into account all absorption, all re-emission, all collisions, all heating and cooling and all eventual loss to space, at all levels.
If the amount of radiation lost to space is equal to the amount coming in from the Sun, Earth is said to be in energy balance. But if the strength of the greenhouse effect is increased, the amount of energy escaping falls behind the amount that is incoming. Earth is then said to be in an energy imbalance and the climate heats up. Double the CO2 concentration and you get a few degrees of warming: double it again and you get a few more and on and on it goes. There is no room for complacency here. By the time just one doubling has occurred, the planet would already be unrecognisable. The insulation analogy in the myth is misleading because it over-simplifies what happens in the atmosphere.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
This myth relies on the use of a word – saturated. When we think of saturated in everyday use, the term 'soggy' comes to mind. This is a good example of a word that has one meaning in common parlance but another very specific one when thinking about atmospheric physics. Other such words come to mind too. Absorb and emit are two good examples relevant to this topic and we’ll discuss how they relate to atmospheric processes below.
First things first. The effect of CO2 in the atmosphere is due to its influence on the transport of 'electromagnetic radiation' (EMR). EMR is energy that is moving as x-rays, ultraviolet (UV) light, visible light, infrared (IR) radiation and so on (fig. 1). Radiation is unusual in the sense that it contains energy but it is also always moving, at the speed of light, so it is also a form of transport. Radiation is also unusual in that it has properties of particles but also travels with the properties of waves, so we talk about its wavelength.
The particles making up radiation are known as photons. Each photon contains a specific amount of energy, and that is related to its wavelength. High energy photons have short wavelengths, and low energy photons have longer wavelengths. In climate, we are interested in two main radiation categories - firstly the visible light plus UV and minor IR that together make up sunshine, and secondly the IR from the earth-atmosphere system.
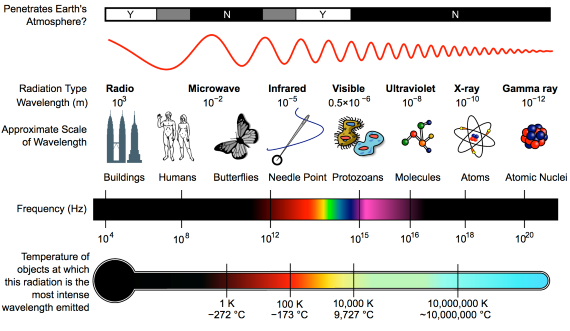
Fig. 1: diagram showing the full electromagnetic spectrum and its properties of the different bands. Image: CC BY-SA 3.0 from Wikimedia.
CO2 has the ability to absorb IR photons – it is a 'greenhouse gas'.So what does “absorb” mean, when talking about radiation? We are all familiar with using a sponge to mop up a water spill. The sponge will only absorb so much and will not absorb any more unless it's wrung out. In everyday language it may be described, without measurements, as 'saturated'. In this household example, 'absorb' basically means 'soak up' and 'saturated' simply means 'full to capacity'. Scientific terms are, in contrast, strictly defined.
Now let's look at the atmosphere. The greenhouse effect works like this: energy arrives from the sun in the form of visible light and ultraviolet radiation. A proportion reaches and warms Earth's surface. Earth then emits the energy in the form of photons of IR radiation.
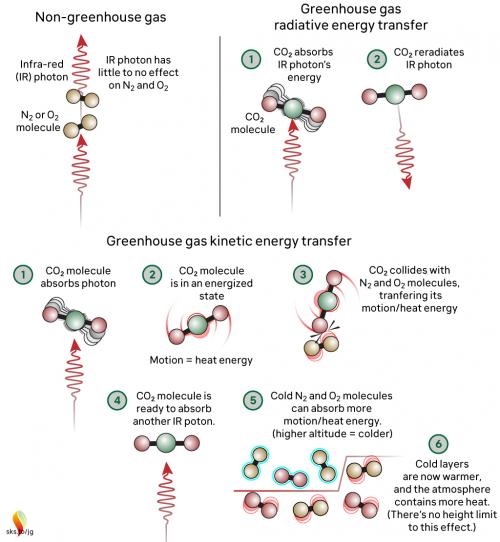
Greenhouse gases in the atmosphere, such as CO2 molecules, absorb some of this IR radiation, then re-emit it in all directions - including back to Earth's surface. The CO2 molecule does not fill up with IR photons, running out of space for any more. Instead, the CO2 molecule absorbs the energy from the IR photon and the photon ceases to be. The CO2 molecule now contains more energy, but that is transient since the molecule emits its own IR photons. Not only that: it's constantly colliding with other molecules such as N2 and O2 in the surrounding air. In those collisions, that excess energy is shared with them. This energy-sharing causes the nearby air to heat up (fig. 2).
Fig. 2: The greenhouse effect in action, showing the interactions between molecules. The interactions happen at all levels of the atmosphere and are constantly ongoing. Graphic: jg.
The capacity for CO2 to absorb photons is almost limitless. The CO2 molecule can also receive energy from collisions with other molecules, and it can lose energy by emitting IR radiation. When a photon is emitted, we’re not bringing a photon out of storage - we are bringing energy out of storage and turning it into a photon, travelling away at the speed of light. So CO2 is constantly absorbing IR radiation, constantly emitting IR radiation and constantly sharing energy with the surrounding air molecules. To understand the role of CO2, we need to consider all these forms of energy storage and transport.
So, where does 'saturation' get used in climate change contrarianism? The most common way they try to frame things is to claim that IR emitted from the surface, in the wavelengths where CO2 absorbs, is all absorbed fairly close to the surface. Therefore, the story continues, adding more CO2 can’t make any more difference. This is inaccurate through omission, because either innocently or deliberately, it ignores the rest of the picture, where energy is constantly being exchanged with other molecules by collisions and CO2 is constantly emitting IR radiation. This means that there is always IR radiation being emitted upwards by CO2 at all levels in the atmosphere. It might not have originated from the surface, but IR radiation is still present in the wavelengths that CO2 absorbs and emits. When emitted in the upper atmosphere, it can and will be lost to space.
When you include all the energy transfers related to the CO2 absorption of IR radiation – the transfer to other molecules, the emission, and both the upward and downward energy fluxes at all altitudes - then we find that adding CO2 to our current atmosphere acts to inhibit the transfer of radiative energy throughout that atmosphere and, ultimately, into space. This will lead to additional warming until the amount of energy being lost to space matches what is being received. This is precisely what is happening.
The myth reproduced at the top – incorrectly stating an analogy with roof insulation in that each unit has less of an effect - is misleading. Doubling CO2 from 280 ppm to 560 ppm will cause a few degrees of warming. Doubling again (560 to 1130 ppm) will cause a similar amount of additional warming, and so on. Many doublings later there may be a point where adding more CO2 has little effect, but recent work has cast serious doubt on that (He et al. 2023). But we are a long, long way from reaching that point and in any case we do not want to go anywhere near it! One doubling will be serious enough.
Finally, directly observing the specific, global radiative forcing caused by well-mixed greenhouse gases has - to date - proven elusive. This is because of irregular, uncalibrated or limited areal measurements. But very recently, results have been published regarding the deep reinterrogation of years of data (2003-2021) from the Atmospheric Infrared Sounder (AIRS) instrument on NASA's Aqua Satellite (Raghuraman et al. 2023). The work may well have finally cracked the long-standing issue of how to make finely detailed, consistent wavelength-specific measurements of outgoing long-wave radiation from Earth into space. As such, it has opened the way to direct monitoring of the radiative impact (i.e. forcing + feedback) of greenhouse gas concentration changes, thereby complimenting the Keeling Curve - the longstanding dataset of measured CO2 concentrations, down at the planet's surface.
Note: Several people in addition to John Mason were involved with updating this basic level rebuttal, namely Bob Loblaw, Ken Rice and John Garrett (jg).
Last updated on 31 December 2023 by John Mason. View Archives































 Arguments
Arguments





































Bob Loblaw@724
Above the extinction altitude of the 15 micron band, CO2 can still emit IR radiation (at any wavelength) but can no longer absorb within this band. The fact that CO2 can no longer absorb within this band means that it has zero greenhouse forcing at this altitude and above for the simple reason that there is no more 15 micron radiation that can be absorbed. The small amount of 15 micron energy that reaches orbital sensors comes from blackbody emissions from the top of the credible atmosphere. This radiation cannot, however, be absorbed since there is no more atmosphere.
CallItAsItIs @ 726:
Frankly. you don't know what you are talking about.
I know this seems like a difficult concept for you to grasp, but the atmosphere has more than just a top and a bottom. In fact, almost all of the "credible atmosphere" exists in the zone between the top and the bottom, and until you learn that you will continue to spout garbage.
CallItAsItIs @726 (and prior) :-
You are expressing yourself in an unclear manner ~ almost bizarrely.
e.g. What is the term "extinction altitude" that you use?
And why do you say that a CO2 molecule "can no longer absorb" (at the 15 micron wavelength) when the molecule is at say 50,000m altitude versus when the molecule is at say 50m or 500m altitude? Please explain.
First, thanks to Bob for the call out. Studying the post and the linked article in Chemical Engineering Progess should be an effective means of education.
CallitAsitis @726:
You have a misunderstanding of Kirchhoff’s Law: Absorptance = Emittance (at thermal equilibrium, i.e., temperature not changing) A CO2 molecule absorbs a photon related to a specific wavelength, increasing its internal energy level. It collides with adjacent N2 and O2 molecules and transfers energy so that the molecules are in thermal equilibrium. Since CO2 still vibrates, it also emits photons at specific wavelengths. For the molecule, the net result is essentially a pass-through. For a column of atmosphere, it is not conservation of photons but conservation of energy.
You also have a misunderstanding of the absorptance/emittance lines for CO2 and water vapor. See Figure 3 in the post linked by Bob. The Stefan-Boltzmann Law is applied to each line:
Intensity = emittance x Stefan-Boltzmann constant x absolute temperature to the 4th power.
Your understanding of absorptance/emittance as a function of altitude is not correct. Energy loss to space is best conceptualized by looking down from the top of the atmosphere, not by looking up from the surface. Looking down, Beer’s Law determines the altitude at which there are sufficient molecules in the optical path to bring the emittance to 1.0. For strong CO2 emittance lines, it is the cold, thin atmosphere at the bottom of the stratosphere. For weak emittance lines, it is at a lower, thicker, warmer altitude, with a minimum altitude being the Earth’s surface which emits as a blackbody. As CO2 increases, the molecular density in the cold upper atmosphere increases, the weak emittance lines strengthen, and intensity of emitted energy decreases because of the lower temperature.
Eclectic @728
The extinction altitude of an absorption band of a GHG is the altitude at which the upwelling radiation with the band becomes negligible according to the Beer-Lambert law and the absorption coefficient of the band. For CO2, the absorption band is 14-16 microns and the extinction altitude is about 10 meters. This means the upwelling IR radiation absorbed by CO2 at the top of the credible atmosphere is pretty miniscule. Above that, of course, it is zero.
Regarding the CO2 molecule at 50000m, it most certainly can absorb the 15 micron IR photons — if you bring an IR source up there. The reason there is no absorption at that altitude is because all 15 micron IR has already been absorbed at lower altitudes.
To show how CallItAsItIs is wrong, let's look at the upward-directed IR radiation calculated using the MODTRAN model I mentioned in comment 725. We'll stick to the default tropical atmosphere with no clouds - changing only the altitude we're looking down from.
Let's start at 10km altitude.
Lots of radiation at 15um there. Haven't reached "extinction" yet. Lets' go higher, to 20km...
A substantial reduction in 15um intensity. Are we close to CallItAsItIs's "extinction" height yet? Let's go higher, to 30km...
Oh, no! IR intensity at 15um has increased! How on earth is that possible? Where are those 15um photons coming from? If they were blocked from reaching 20km (from the surface), then how can they possibly be appearing at 30km altitude? At 30km, we're now in the stratosphere and the atmosphere is getting warmer again. Could it be possible that this warmer atmosphere is actually emitting 15um photons?
Maybe we need to go higher. Try 50 km.
Oh, crap. 15um intensity is even higher. Let's try 70km.
That's almost the same as at 50km.
So, where is this "extinction" point where CallItAsItIs claims there will be no more 15um IR radiation for CO2 to absorb? That "extinction" point only exists in CallItAsItIs's imagination.
CallItAsItIs @ 730 once again completely ignores the known and measured fact that upwelling IR radiation at 15um, as measured at high altitudes, does not need to come from the surface.
He even has the answer in his comment (emphasis added):
Please buy a clue, CallItAsItIs. We do not need to "bring an IR source up there" - there already is one. It's called "the upper atmosphere", it contains CO2, and it is warmer than 0K. Climate scientists actually know about this obscure "upper atmosphere" as a source of IR radiation, they know how to calculate its effect, and they know it plays a role in atmospheric greenhouse warming.
I noticed that CallItAsItIs, in comment 730, has stated that his "extinction height" is a mere 10m. If this actually prevented there from being any upwelling 15um radiation above that height, then all the graphs I supplied in comment 731 should show zero for intensity at 15um.
The astute observer will note that the graphs do no such thing.
CallItAsItIs :-
Your explanation falls short of reality.
At the planetary scale, the "Beer-Lambert Law" is not germane to the situation (see Bob Loblaw's further comments, above).
Always beware of "Laws" composed centuries ago. The so-called Laws were useful as mental short-cuts, in some but not all circumstances.
As Einstein would say: Take your nose out of the lawyers' books, and look at the real universe.
Eclectic @ 734
The fundamental laws of radiant energy, including Beer’s Law, are entirely germane to the mechanism of global warming. They are not mental shortcuts. Einstein was in another league when thinking about the theory of relativity.
The problem for CallitAsItIs is that he does not apply Beer’s Law correctly as it applies to radiant energy leaving the top of the atmosphere. He views, incorrectly, attenuation of the original source radiation from the surface, calling it “extinction.” This is the same mistake that Angström made in 1900 and too many others have followed since because it overlooks Kirchhoff’s Law. Bob Loblaw gets it right because he uses the MILIA (MODTRAN Infrared Light in the Atmosphere) model looking down from the top of the atmosphere at 70 km. Beer’s Law is very important because it defines the molecular density in the atmosphere that raises the emittance of a specific wavelength to a value of 1.0. Note that the minimum atmospheric temperature for the Tropical Atmosphere is 195 Kelvin at 17 km. The reason the bottom of the CO2 trough begins to rise at higher altitudes is the increasing temperature of the ozone layer. This demonstrates clearly that there are sufficient CO2 molecules, by Beer’s Law, to form an emitting layer at that altitude.
It is enlightening to run the model at 10 meters looking down. The spectra follows the Planck distribution because all source photons from the surface that are absorbed by CO2 are re-emitted. Kirchhoff's Law.
CallItAsItIs - posters above are trying to educate you about how to work with atmospheric physics but so far you appear to be very reluctant to understand the points made.
Can we agree as a starting point that nature has the final say? Ie the basis of science. You are proposing a, let's say, novel hypothesis for physics where as climate science is using the long established model of radiative transfer. That model allows us to predict what instruments on ground, balloon, satellite will measure for spectrum (and many other things as well). Your hypothesis would give very different observations.
Are you prepared to let the observations decide the argument?
@735 :-
Thank you, Charlie_Brown, but please look to the main thrust of my comment.
Which is that: regardless of Beer's Law, Bouguer's Law, Lambert's Law, Kirchhoff's Law, Thermodynamics Law(s), etcetera . . . we must not get in the rather lazy habit of accepting famous "Laws" in an automatic way ~ by taking the legalistic approach that holds that words & concepts are equivalent to actual physical reality (rather than as sometimes convenient guides).
I'm sure you have seen that sort of thinking quite often with Climate Science Deniers who assert that "a colder thing cannot warm a warmer thing" (and so on).
Sometimes the old "Laws" are fine in most circumstances; sometimes they are useful as approximations ~ but "Laws" are essentially concepts rather than hard realities of particles/photons. And they can ~ sometimes ~ mislead our thinking. Beware of Laws.
Electic:
The issue with Beer's Law is not that it is incorrect - it's that it is incomplete. It only deals with the absorption side of the radiative transfer process. It says nothing at all about the emission side. To properly describe and understand the greenhouse effect and IR radiative fluxes (upwards and downwards), you need both. Beer's Law only gets you half way there.
It's kind of like trying to balance your bank book by adding up all the deposits and ignoring the withdrawals (or vice versa).
There is useful discussion of Beer's Law on this "From the email bag" post (now almost three years old). In particular, read the first few comments where Charlie Brown and I start to discuss the "extras" needed to complete the picture.
Eclectic @737,
I strongly disagree with your description of the laws of science. The definition is not like legalistic law. Understanding and applying them properly is in no way lazy. They are not concepts. In the scientific use of the word, they are physical reality. The laws of gravity, conservation of energy, the 2nd law of thermodynamics, and radiant energy transfer have never been found to have been violated. But it does take knowldege and careful thought to apply them correctly. It is like the old cliché about statistics never lie but liars use statistics. Statistics are rigorous but too many people use them improperly.
Yes, I know the thread about the 2nd law of thermo about a colder thing cannot warm a warmer thing. I answered the issue with Gerlich & Tscheusner’s paper @1535 by pointing out that G&T made a mistaken assumption that the mechanism of global warming was “radiatively equilibrated.” Since global warming results from an upset to steady state equilibrium, there is no violation of the 2nd law. Understanding the law and G&T mistaken assumption should have put a stop to that myth.
In this case, Beer’s Law (I use that term now as Beer focused on concentration while Lambert focused on column length, but both convey the same concept, the atmosphere essentially has a fixed column length) is only one part of the mechanism of global warming. As I tried to say very clearly to CallItAsItIs, he errored by not applying Kirchhoff’s Law. Anyone who doesn’t understand it should study it before making comments before spreading misinformation. Errors made by science denialists need to be found and explained. I think that using the laws of science is the best way to be convincing. The challenge is to find the errors and explain them in understandable terms.
Bob Loblaw @ 731
I hate to disappoint you, but your curves tend to show that I am right. As the observation altitude increases, the intensity at 15 microns approaches a value corresponding to a 220 K blackbody. This, in turn, corresponds to an altitude of about 70 km, above which there isn't much of an atmosphere. Therefore, for the 15 micron band, the detectors are only picking up some thermal radiation from the TOA. Any upwelling radiation from this band has already been completely absorbed at lower altitudes.
Bob Loblaw @732
Regarding your comment
Please buy a clue, CallItAsItIs. We do not need to "bring an IR source up there" - there already is one. It's called "the upper atmosphere", it contains CO2, and it is warmer than 0K. Climate scientists actually know about this obscure "upper atmosphere" as a source of IR radiation, they know how to calculate its effect, and they know it plays a role in atmospheric greenhouse warming.
Are you saying that the atmosphere heats itself!? Wrong! This would violate energy conservation. In order to to heat the atmosphere, we must bring in IR from outside the atmosphere. In the case of greenhouse warming, this is the 288 K IR eminating from the surface of the earth.
Charlie_Brown @739 :-
Our two-way conversation is getting rather off-topic for this thread.
I am sure that we two are "on the same side" regarding AGW/science. But there is clearly a large semantic discrepancy with our individual understandings of the meaning of certain English words ~ and it would take far too long for us to negotiate a mutual agreement on the semantics.
Let it slide.
The point which I found interesting (and which I always find interesting in reading all the nonsenses, bad science, and Motivated Reasonings to be found on the WUWT website) . . . is that our new friend [viewed @722 ; @723 ; @726 ; @730 ; @740 ; and @741 ] and suchlike people ~ are coming to pseudo-science conclusions because of poor logic and poor understanding of the physical universe of particles & photons. They are hampered by their confusions of realities vs abstractions (e.g. the mental constructions achieved by the great scientists of the 18th, 19th, and later centuries. Constructions which have been dignified by the label "Laws" ) .
[ Off-hand, I do not know of a suitable thread on SkS , for the discussion of the philosophy of science as expressed through human psychology & semantics. ]
CallItAsItIs @ 740:
Are you actually paying attention to what you are saying?
The level of physics denial is astounding. For your explanation to be correct, the entire atmosphere between the lower near-surface layers and the top of atmosphere layers cannot emit IR radiation in the 15um band. In your version of "physics":
You are creating a physics-free zone between the surface and the TOA. In spite of the fact that the entire atmosphere has a temperature above 0K, and therefore contains thermal energy, and therefore emits thermal radiation (including 15um radiation, because the CO2 that is present can do that...), you have declared that none of that physics applies and none of the upwelling 15um radiation that is measured between the surface and the TOA exists.
...and then in comment 741, you wander off into a tangent claiming that my explanation in comment 732 requires that the atmosphere "heats itself" and "violate[s] energy conservation". That is more physics denial. Any object with a temperature >0K contains thermal energy. And the laws of physics state that any object >0K will emit radiation.
When a molecule emits radiation, it loses energy (conservation of energy maintained) and will cool, but each molecule is capable of regaining energy by collisions with other molecules, or by absorbing radiation (in any wavelength - the absorption and emission do not need to be in the same wavelength).
Remember in comment 722 when you said?:
The opposite is also true. CO2 can gain energy from N2 and O2 through collisions, when the N2 and O2 molecules are at higher thermal energy levels than the CO2. That is what predominantly drives the "thermal radiation" emitted by CO2 at all levels in the atmosphere. You can read the technical details in this post at Eli Rabett's blog.
To try to make a few points, independent of CallItAsItIs's confusion:
To call it as it is, it appears that CallItAsItIs fails to understand many of these essential aspects of physics and radiation transfer.
Bob’s points are spot on. CallItAsItIs sometimes uses Kirchhoff’s Law and sometimes forgets about it. There is one more interesting point that I would like to add about interpreting the graphs. They demonstrate conclusively that there are sufficient CO2 molecules to form an emitting layer (emittance = 1.0) at the bottom of the stratosphere. This shows the value of interpreting Beer’s Law by looking down from the top of the atmosphere.
CallItAsItIs misses the interpretation when he says “the intensity at 15 microns approaches a value corresponding to a 220 K blackbody. This, in turn, corresponds to an altitude of about 70 km, above which there isn't much of an atmosphere.” 220 K also corresponds to an altitude of 24 km and that is the source of radiant energy loss to space in the 14-16 micron band. How do I know? Look at the 15-micron peak, which is composed of a few very strong emittance lines, in the spectrum at 50 km. The temperature at that altitude is 270 K, but the top of the peak corresponds to the Planck distribution of 241 K. By Beer's Law, there are not enough CO2 molecules to bring the emittance to 1.0 for the strongest peak.
I feel as if I'm on the verge of beating a dead horse - no idea if CallItAsItIs will respond to the latest comments, but....
I think it is useful to provide a graphic published in a 1967 paper by Manabe and Wetherald.
Figure 16 looks like this:
Note that increasing CO2 increases surface temperatures, but leads to cooling in the stratosphere. Why? Many factors, but one of them is that by adding CO2, the overall emissivity of the atmosphere increased - so the atmosphere can emit the same IR radiation to space at a lower temperature. Kirchoff's Law at work - both absorptivity and emissivity change in unison.
But CallItAsItIs has not yet caught up with science from 1967, so he believes that only the emission of "thermal radiation" is being picked up by satellite sensors, and there is no emission from CO2.
Regarding Charlie Brown's comment at 745 (and earlier) about thinking of Beer's Law from the perspective of the top of the atmosphere:
As we follow the 15um flux up from the surface, Beer's Law tells us that at each height, we're probably looking at more photons that were emitted at heights slightly below where we are, and fewer photons that were emitted far, far below where we are. There will always be 15um photons flowing upward, though.
Charlie_Brown @745
Could you please explain to me how my postings are not consistent with Kirchhoff's Law?
Bob Loblaw @ 744
From this comment and several others of yours, I believe we need to get some basics straight so that you and I are consistent in our use of various term (eg. thermal radiation). Also, we need to be certain we are even working the same problem. Although I am addressing this message specifically to you, Charlie_Brown, Eclectic, and scaddenp are certainly welcome to tune in.
In this problem, we are trying to assess warming of the atmosphere due to IR radiation eminating from the surface of the earth. This earth-emitting IR is estimated as a blackbody at about 288 deg. K, although we do consider it to be adjustable.
For energy conservation, we must take this to be the only source of energy causing addtional warming to the entire atmosphere. This does not mean, however, that energy can't change forms within the atmosphere. Indeed, this is what happens in establishing thermal equilibrium. But there cannot be any change in total energy in the process. The atmosphere does not consume fuel and has no means of generating new energy.
Next, we assume that the molecules and photons are of sufficient number that it makes sense to talk about smoothly varying densities, fluid velocities, temperatures, and pressures. Also, we assume that thermal equilibrium is maintained at least locally. This is what enables us to discuss different temperatures at different altitudes.
The term thermal radiation is used to denote the distribution of EMR when thermal equilbrium is reached. If the atmosphere was totally absorptive at all EMR frequencies, then the thermal radiation would simply be given by the Planck distribution. Of course this is not the case for the entire EMR spectrum, but at wavelengths near 15 microns or other bands that are strongly absorptive, the atmospheric radiance at those wavelengths comes close to matching those of a blackbody at the temperature where the measurement is taken.
Applying this concept of thermal radiation to the TOA (about 70km altitude), we find that CO2 in effect forms a narrow bandwidth "blackbody" at the 15 micron wavelength. At this point, the detectors pick up on the emissions from this "blackbody" since there is no more CO2 at the TOA to attenuate them. This is why the last three curves that Bob Loblaw posted in 731 all show the same intensity (about 6 W/m^2) and the same temperature (about 220 deg. K) of the 15 micron band. Emissions from this band at lower altitudes and higher temperatures have all been absorbed.
At this point, we still need to discuss absorption coefficients and the Beer Lambert equation which govern the conversion of upwelling IR radiation from the surface to thermal energy of the atmosphere, but I think I will wait for feedback before I spend any more time at this.
CallItAsItIs @749 :-
Please do not forget about the Sun warming the atmosphere.
Also, convection and H2O phase changes, etc.
I'm fairly sure you were going to mention all these other factors, and also bring Trenberth's summary into the discussion. After all, we would be remiss if we ignored the Big Picture and focused entirely on only IR photons and the abstract "Laws of Physics".