Is the CO2 effect saturated?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
The notion that the CO2 effect is 'saturated' is based on a misunderstanding of how the greenhouse effect works. |
|||||||
Climate Myth...
CO2 effect is saturated
"Each unit of CO2 you put into the atmosphere has less and less of a warming impact. Once the atmosphere reaches a saturation point, additional input of CO2 will not really have any major impact. It's like putting insulation in your attic. They give a recommended amount and after that you can stack the insulation up to the roof and it's going to have no impact." (Marc Morano, as quoted by Steve Eliot)
At-a-Glance
This myth relies on the use (or in fact misuse) of a particular word – 'saturated'. When someone comes in from a prolonged downpour, they may well exclaim that they are saturated. They cannot imagine being any wetter. That's casual usage, though.
In science, 'saturated' is a strictly-defined term. For example, in a saturated salt solution, no more salt will dissolve, period. But what's that got to do with heat transfer in Earth's atmosphere? Let's take a look.
Heat-trapping by CO2 in the atmosphere happens because it has the ability to absorb and pass on infra-red radiation – it is a 'greenhouse gas'. Infra-red is just one part of the electromagnetic spectrum, divided by physicists into a series of bands. From the low-frequency end of the spectrum upwards, the bands are as follows: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Gamma rays thus have a very high-frequency. They are the highest-energy form of radiation.
As our understanding of the electromagnetic spectrum developed, it was realised that the radiation consists of particles called 'photons', travelling in waves. The term was coined in 1926 by the celebrated physicist Gilbert Lewis (1875-1946). A photon's energy is related to its wavelength. The shorter the wavelength, the higher the energy, so that the very high-energy gamma-rays have the shortest wavelength of the lot.
Sunshine consists mostly of ultraviolet, visible light and infra-red photons. Objects warmed by the sun then re-emit energy photons at infra-red wavelengths. Like other greenhouse gases, CO2 has the ability to absorb infra-red photons. But CO2 is unlike a mop, which has to be wrung out regularly in order for it to continue working. CO2 molecules do not get filled up with infra-red photons. Not only do they emit their own infra-red photons, but also they are constantly colliding with neighbouring molecules in the air. The constant collisions are important. Every time they happen, energy is shared out between the colliding molecules.
Through those emissions and collisions, CO2 molecules constantly warm their surroundings. This goes on all the time and at all levels in the atmosphere. You cannot say, “CO2 is saturated because the surface-emitted IR is rapidly absorbed”, because you need to take into account the whole atmosphere and its constant, ongoing energy-exchange processes. That means taking into account all absorption, all re-emission, all collisions, all heating and cooling and all eventual loss to space, at all levels.
If the amount of radiation lost to space is equal to the amount coming in from the Sun, Earth is said to be in energy balance. But if the strength of the greenhouse effect is increased, the amount of energy escaping falls behind the amount that is incoming. Earth is then said to be in an energy imbalance and the climate heats up. Double the CO2 concentration and you get a few degrees of warming: double it again and you get a few more and on and on it goes. There is no room for complacency here. By the time just one doubling has occurred, the planet would already be unrecognisable. The insulation analogy in the myth is misleading because it over-simplifies what happens in the atmosphere.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
This myth relies on the use of a word – saturated. When we think of saturated in everyday use, the term 'soggy' comes to mind. This is a good example of a word that has one meaning in common parlance but another very specific one when thinking about atmospheric physics. Other such words come to mind too. Absorb and emit are two good examples relevant to this topic and we’ll discuss how they relate to atmospheric processes below.
First things first. The effect of CO2 in the atmosphere is due to its influence on the transport of 'electromagnetic radiation' (EMR). EMR is energy that is moving as x-rays, ultraviolet (UV) light, visible light, infrared (IR) radiation and so on (fig. 1). Radiation is unusual in the sense that it contains energy but it is also always moving, at the speed of light, so it is also a form of transport. Radiation is also unusual in that it has properties of particles but also travels with the properties of waves, so we talk about its wavelength.
The particles making up radiation are known as photons. Each photon contains a specific amount of energy, and that is related to its wavelength. High energy photons have short wavelengths, and low energy photons have longer wavelengths. In climate, we are interested in two main radiation categories - firstly the visible light plus UV and minor IR that together make up sunshine, and secondly the IR from the earth-atmosphere system.
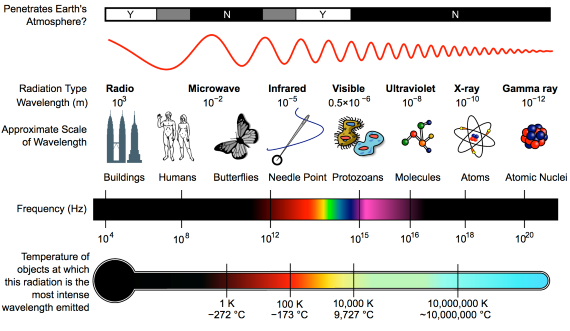
Fig. 1: diagram showing the full electromagnetic spectrum and its properties of the different bands. Image: CC BY-SA 3.0 from Wikimedia.
CO2 has the ability to absorb IR photons – it is a 'greenhouse gas'.So what does “absorb” mean, when talking about radiation? We are all familiar with using a sponge to mop up a water spill. The sponge will only absorb so much and will not absorb any more unless it's wrung out. In everyday language it may be described, without measurements, as 'saturated'. In this household example, 'absorb' basically means 'soak up' and 'saturated' simply means 'full to capacity'. Scientific terms are, in contrast, strictly defined.
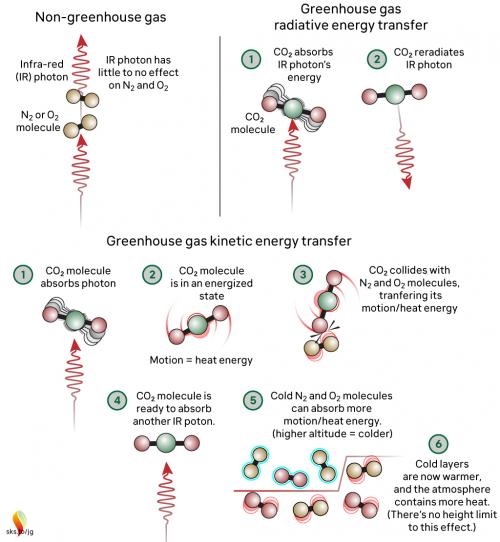
Now let's look at the atmosphere. The greenhouse effect works like this: energy arrives from the sun in the form of visible light and ultraviolet radiation. A proportion reaches and warms Earth's surface. Earth then emits the energy in the form of photons of IR radiation.
Greenhouse gases in the atmosphere, such as CO2 molecules, absorb some of this IR radiation, then re-emit it in all directions - including back to Earth's surface. The CO2 molecule does not fill up with IR photons, running out of space for any more. Instead, the CO2 molecule absorbs the energy from the IR photon and the photon ceases to be. The CO2 molecule now contains more energy, but that is transient since the molecule emits its own IR photons. Not only that: it's constantly colliding with other molecules such as N2 and O2 in the surrounding air. In those collisions, that excess energy is shared with them. This energy-sharing causes the nearby air to heat up (fig. 2).
Fig. 2: The greenhouse effect in action, showing the interactions between molecules. The interactions happen at all levels of the atmosphere and are constantly ongoing. Graphic: jg.
The capacity for CO2 to absorb photons is almost limitless. The CO2 molecule can also receive energy from collisions with other molecules, and it can lose energy by emitting IR radiation. When a photon is emitted, we’re not bringing a photon out of storage - we are bringing energy out of storage and turning it into a photon, travelling away at the speed of light. So CO2 is constantly absorbing IR radiation, constantly emitting IR radiation and constantly sharing energy with the surrounding air molecules. To understand the role of CO2, we need to consider all these forms of energy storage and transport.
So, where does 'saturation' get used in climate change contrarianism? The most common way they try to frame things is to claim that IR emitted from the surface, in the wavelengths where CO2 absorbs, is all absorbed fairly close to the surface. Therefore, the story continues, adding more CO2 can’t make any more difference. This is inaccurate through omission, because either innocently or deliberately, it ignores the rest of the picture, where energy is constantly being exchanged with other molecules by collisions and CO2 is constantly emitting IR radiation. This means that there is always IR radiation being emitted upwards by CO2 at all levels in the atmosphere. It might not have originated from the surface, but IR radiation is still present in the wavelengths that CO2 absorbs and emits. When emitted in the upper atmosphere, it can and will be lost to space.
When you include all the energy transfers related to the CO2 absorption of IR radiation – the transfer to other molecules, the emission, and both the upward and downward energy fluxes at all altitudes - then we find that adding CO2 to our current atmosphere acts to inhibit the transfer of radiative energy throughout that atmosphere and, ultimately, into space. This will lead to additional warming until the amount of energy being lost to space matches what is being received. This is precisely what is happening.
The myth reproduced at the top – incorrectly stating an analogy with roof insulation in that each unit has less of an effect - is misleading. Doubling CO2 from 280 ppm to 560 ppm will cause a few degrees of warming. Doubling again (560 to 1130 ppm) will cause a similar amount of additional warming, and so on. Many doublings later there may be a point where adding more CO2 has little effect, but recent work has cast serious doubt on that (He et al. 2023). But we are a long, long way from reaching that point and in any case we do not want to go anywhere near it! One doubling will be serious enough.
Finally, directly observing the specific, global radiative forcing caused by well-mixed greenhouse gases has - to date - proven elusive. This is because of irregular, uncalibrated or limited areal measurements. But very recently, results have been published regarding the deep reinterrogation of years of data (2003-2021) from the Atmospheric Infrared Sounder (AIRS) instrument on NASA's Aqua Satellite (Raghuraman et al. 2023). The work may well have finally cracked the long-standing issue of how to make finely detailed, consistent wavelength-specific measurements of outgoing long-wave radiation from Earth into space. As such, it has opened the way to direct monitoring of the radiative impact (i.e. forcing + feedback) of greenhouse gas concentration changes, thereby complimenting the Keeling Curve - the longstanding dataset of measured CO2 concentrations, down at the planet's surface.
Note: Several people in addition to John Mason were involved with updating this basic level rebuttal, namely Bob Loblaw, Ken Rice and John Garrett (jg).
Last updated on 31 December 2023 by John Mason. View Archives































 Arguments
Arguments





































Just to let you all know that I now have some equations on scratch paper. My solution uses Schwartzschild's equation along with the solution from the Wikipedia article previously mentioned. Also, I do not use Kirchhoff's Law since I showed in comment 800 that it does not apply in the case of greenhouse warming.
Now, at this point, the only way I can typeset equations is with LaTex, and after writing the .tex files, I can print them to .pdf files. What would be easiest for me is to simply submit the .pdf file in a manner you specify, and let you decide how to handle it.
Finally, please realize that this is not a trivial effort on my part. Therefore, please speakup now if you object to any aspect of the model I am setting up. Understand that it is one thing to review my work and find errors. Claiming that I am "re-inventing physics", however, is quite another matter and I will be in no mood for hearing it.
The only option you have for including graphics is to place a jpeg on a public host of some sort, and then include the link when inserting an image as you compose your comment. That image file can be from a scan or screen grab or exported from whatever software you want. The essential characteristic is that you place it in a publicly-visible web server.
In the past, some people have used https://tinypic.host. I have no idea if it still works. It is up to you to find a suitable host. The use of graphics is explained in the Comments Policy.
Given that Kirchoff's Law is an essential assumption behind the Schwartzschild equation (as has been explained to you previously), let it be known that any comment that claims to use the Schwartzschild equation without the use of Kirchoff's Law will be considered to be in error.
We are in no mood for hearing more of your egregious misunderstandings of conventional physics. You may not get more than one shot at this.
Just to let you all know that I have some equations and results on scratch paper that you may be interested in seeing. And they show exactly what MA Rodger requests in the last paragraph of comment 849. It will probably take a few days, however, for me the get it pulled together in a presentable fashion.
At this point, the only way I know to typeset equations is with LaTex. So, I could write a .tex file for the solution document, print it to a .pdf file, and e-mail or ftp the file to where you want it. Or, I could send you the .tex file itself. It would definitely take more time and coaching for me to submit it directly from the Post-a-Comment form.
So let me know how you want to proceed with this. You can use the email address I used for subscribing if you want to contact me without cluttering this webpage. I hope to hear from you soon.
[PS] You can only create a link to the PDF somewhere (eg via onedrive or googledrive), or insert an image of the equations. See the comment policy link for hints on posting images.
I'm not entirely sure why a mathematical equation requires an image to be displayed. It can be clearer when not set out as a linear string of characters and some exotic characters may not be available in extended font sets, but such restrictions are hardily show-stoppers.
But if that is the way to go and an on-line image is felt necessaary, I uploaded the thumbnail below at https://postimages.org/ which required just an indication of size and longevity (which can be "No expiration") to be up-loaded, the thunbnail below having a 31-day on-line life. So, does it work?
"Does it work?"
Yes!
Callitasit is:
YOur comment at 800 does not mention Kirchhoff's law so you have not "showed in comment 800 that it does not apply in the case of greenhouse warming." You need to show that now if you want to claim it is the case.
Think about it. You say that you did not understand that Kirchhoffs law did not apply to the atmosphere until you engaged in this discusssion. Since this is an important law to consider (even if it did not apply) in the transission of IR energy it is clear that you do not understand a lot about how energy is transmitted through the atmosphere. Do you really think you can show that thousands of atmospheric scientists are wrong when you do not undersatnd the basics of atmospheric energy?
Please do not encourage CallItAsItIs to engage in further side discussions of small parts of his viewpoint. Our requirement for his continued engagement here is for him to provide a full description of his equations for radiative flux through a layer. (Ideally, he'd provide a full description of all energy fluxes through a layer, but let's try to get radiative flux first.)
The hope is that by "putting it all together", he will realize the many inconsistencies he demonstrates when treating small parts of the issue in isolation.
As a prime example of his inconsistency, consider his statement in comment 851 (emphasis added):
He has repeatedly said that Kirchhof's Law does not apply when we do not have "thermal equilibrium". This is not what any legitimate source says - they repeatedly use the term "local thermodynamic equilibrium" as a requirement. Wikipedia has a page on thermodynamic equilibrium. That page specifically discusses "local and global equilibrium". In the opening paragraph, we see:
CallitAsItIs has completely failed to explain why this concept of local thermodynamic equilibrium is not a reasonable assumption.
But let's consider what it would mean if this assumption of local thermodynamic equilibrium is not valid, so Kirchhof's Law could not be applied. Let's look at what is said about the Schwarzschild equation (also on Wikipedia). The very first sentence (emphasis added):
Later in the Wikipedia document, it states (emphasis added):
In other words, as long as molecules are gaining and losing energy by collision faster than by absorbing and emitting radiation, local thermodynamic equilibrium is a safe assumption. CallitAsItIs has been previously pointed to this post by Eli Rabett, explain this in detail. He has either not read it, not understood it, or rejected it because he does not recognize why it is important.
...but here is the catch: CallitAsItIs seems quite happy to reject Kirchhoff's Law "because thermal equilibrium", but he does not reject Schwarzschild's equation for the same reason. Even though both of them require an assumption of "local thermodynamic equilibrium".
It's one thing to not understand "local thermodynamic equilibrium", but it is far, far worse to use that misunderstanding selectively. If the argument is valid (it isn't), then it is always valid. It's not "only valid when I want to use it, safe to ignore when inconvenient".
This comes to the meat of CallItAsItIs's misunderstandings. There is no consistency in his arguments and positions. Each inconsistency is a red flag to his misunderstandings.
So, if CallItAsItIs wants to convince anyone, he needs to put all his thoughts on this into one full, coherent, consistent explanation. Once he has written that to his own satisfaction, I hope he will re-read this entire comment thread and reflect on how each criticism he has already received applies to his explanation.
CallitAsItIs seems quite happy to reject Kirchhoff's Law "because thermal equilibrium", but he does not reject Schwarzschild's equation for the same reason. Even though both of them require an assumption of "local thermodynamic equilibrium".
No! What I am rejecting is the climate science version of Kirchhoff's Law which says that for every photon absorbed, an identical one is emitted and vice versa, which is blatantly false! Also, a (false) implication of this "law" is absolutely rigid thermal equilibrium. Therefore, in using the climate science version of Kirchhoff's Law, we are assuming total equilibrium and not just local equilibrium. Additionally, it seems that this "law" is applied regardless of whether or not we have such equilibrium.
So, why is it that climate scientists are using this false version of Kirchhoff's Law? Well, you will have to ask them of course, but here is what I sense is happening. They need the extra photons predicted by this "law" in their model in order to predict CO2 greenhouse warming above what would otherwise be the extinction altitude. Also, it is the basis upon which they make arguments that it is energy exchanges in the higher altitudes rather than near the suface that are important for explaining climate change.
Now, I have explained these things already in previous posts, but you removed them! And it's not fair to delete my explanations and then accuse me of misunderstandings and inconsistencies when in fact I had already addressed them. So please — let's use some discretion about what's deleted so that I don't have to waste time re-posting stuff to answer peoples questions.
Response @855
So, if CallItAsItIs wants to convince anyone, he needs to put all his thoughts on this into one full, coherent, consistent explanation. Once he has written that to his own satisfaction, I hope he will re-read this entire comment thread and reflect on how each criticism he has already received applies to his explanation.
Actually, I've done this already. At this point, I have my arguments and equations pulled together but not yet quite ready for presentation. Over the last few days, I have searched for ways on how I might present this material, but haven't had any luck. BTW, what did you mean in 845 when you stated
Until such time as CallItAsItIS provides a numerical calculation of the purported effects he claims exist, and shows that it agrees with measurements, expect any and all comments from CallItAsItIs or reacting to him to be deleted.
Exactly how am I supposed to provide the material you requested? It would be impossible to post it directly onto this familiar Post-a-Comment page since my equations would have to be handled as images, and I would need at least 1200 pixels of resolution for my equations to render legibly. Post-a-Comment, however, only allows up to 450 pixels. Perhaps it would possible to submit a .pdf file to some hosting company from which SkS could access it, but I simply don't want the hassle of opening and maintaining such an account for a document that probably would not be up for long anyway. What I am willing to do, however, is to write up my results as a .pdf document and email or ftp this .pdf file to an address you provide. You can use the email address associated with my SkS account if you would like to contact me regarding this possibility.
Finally, I should add that I doubt that you or anyone else at SkS will like my results. Basically, I show rigorously from the Schwarzschild equation that I have been correct all along in my claims about CO2 absorption band saturation. Also, I resolved comments made by MA Rodger @849 about some "extra source of excited CO2". So, if you believe I am a crackpot, please send me the appropriate contact info and I will send you a .pdf file of my work so that your "scientific" staff can take some more "pot shots" at it. If, however, you don't want to risk the possibility of bad news about me being right, then don't send the contact info.
[snip]
One last chance. The only post you will be allowed to make is one that explains your "model" in detail. The next non-response to this request will result in your account being blocked. Your tireless, empty assertions of having an alternate explanation for radiative transfer equations will only be believed if you actually present such an explanation, in full.
The only conclusion that we can draw at this point is that you actually cannot provide such an explanation.
Response @856
All right, I'll take that "One last chance", but first I want to be sure we have things straight concerning Kirchhoff's Law. In comment 851 you stated
Given that Kirchoff's Law is an essential assumption behind the Schwartzschild equation (as has been explained to you previously), let it be known that any comment that claims to use the Schwartzschild equation without the use of Kirchoff's Law will be considered to be in error.
Now, as I have already explained (but you deleted), there are two versions of Kirchhoff's Law out there. One violates energy conservation and the other one doesn't, and I will be using the latter of the two. And if that's unacceptable to you, please speak up now! I will not be in a very good mood if I go to all the trouble of pulling this together only to be called a crackpot or accused of "reinventing science".
Alas, you have chosen the path that will not allow you to continue in this forum.
To answer your question: the "second version of Kirchhoff's Law" that you refer to is a figment of your imagination. The fact that you believe in this figment of your imagination (among many) is the reason that you repeatedly fail to present any coherent, self-consistent explanation of atmospheric radiation transfer through a layer.
The fact that you see this figment, in spite of being pointed to many, many sources that explain the proper science to you, is a clear indicator that you way beyond your depth on this subject.
CallItAsItIs @857: Local versus global
Tom Dayton @858
I believe you may have gotten confused as to which "Kirchhoff's Law" we are addressing on this page. What we are talking about is Kirchhoff's Law of Thermal Radiation and not Kirchhoff's circuit laws where your link leads. But thanks for the effort.
CallItAsItIs @ 859: The Moderator @ 855 explained the importance of local versus global. You did not understand. So I gave you an example of local versus global in a different domain, hoping that the different domain would break you out of your narrow perspective so you could understand local versus global more generally. I hoped you would then apply your new understanding of local versus global back to the thermal domain. Apparently I was mistaken.
I'm afraid that the level of compartmentalization in CallItAsItIs's thinking is too strong.
Charlie_Brown @825
CO2 molecules at a specified temperature absorb and emit photons equally, else internal energy would be accumulating and temperature would be changing.
That's just it! Temperature is changing. That's what everyone here is so worried about.
Your eternally-present lack of awareness of context serves to mislead you. In Schwartzschild's equation, for a given rate of absorption, there is one specific temperature (and only one) at which emission and absorption are equal. At any other temperature, they are not. This has been explained to you many times, and you have persisted in misinterpreting and misunderstanding those sources to create a fictional world of "Climate Science as seen by CallItAsItIs".
Charlie_Brown @825
Perhaps I should have made this clearer on my last posting, but your comment
CO2 molecules at a specified temperature absorb and emit photons equally ...
describes a very long-term steady state condition that the atmosphere tends toward on a time-scale of centuries, not years, and certainly not real time. Otherwise we wouldn't be seeing all the ups and downs that have occurred ever since temperatures were first recorded, and there certainly wouldn't be any worries over AGW.
Note to Moderator:
In view of this, could we please move on from holding me to the climate science version of "Kirchhoff's Law" which we know won't happen for a long, long time if ever
Once again, your ignorance betrays you.
CallItAsItIs :-
Allow me to add a naive summation of thermal equilibrium and the many thousands of meter-deep (or centimeter-deep, if your calculation prefers) layers of air that compose the troposphere.
During each 24 hours, in all parts of the planetary troposphere, there are local temperature variations of many degrees Celsius (owing to convectional, advectional, and diurnal changes ).
And yet (A) the vertical transmissions of IR radiation between top & bottom of each tropospheric column are effectuated over the order of 1 second . . . and yet (B) the climatic warming rate for the past half-century is approximately 0.5 degreesC per 30 years.
It follows therefore, that your IR radiation calculations are ~ in practical terms ~ dealing with a thermal equilibrium situation, owing to the brevity of the time window involved (i.e. of 1 second) for neighbouring layers of air.
Your calculation is therefore simple ~ and without the need to get confused or agonize over the presence or absence of "equilibrium".
And yet, over 30 years [ 10 to the 9th power of seconds ] climatic changes are produced by alterations in levels of GreenHouse gasses ~ exactly as has been observed by the scientific studies.
CalItAsItIs has been pointed to Eli Rabett's post on IR absorption and thermal relation several times. If you scroll down in that source, you will find the answer to the time scale at which the energy absorbed from an IR photon is lost via collision to other molecules:
CallItAsItIs had absolutely no understanding of rates of change and how they are related to the various physical processes at play.
CallItAsItIs @862,
May I "call it as it is"?
This becomes farcical.
Whatever the derivation of Schwarzschild's equation, we expect you to use it to demonstrate your proposed IR extinction phenomenon mathematically. I do not see where Kirchoff's Law would be required for such a mathematiacl exercise.
Schwarzschild's equation is expressed thus
dIλ/ds = nσλ[Bλ(t)-Iλ]
where
Bλ(t) = [(2h.c^2)/λ^5] x 1/[e^(hc/kBt -1)]
and we can assume a constant lapse rate thus a linear relationship between t = Ls. And as all of these other factors are constants, the solution is hardily difficult!!
Alas, what we "expect" is for CallItAsItIs to continue to dodge and weave and make excuses to not demonstrate his proposed IR extinction phenomenon mathematically, So his time here at SkS is over.
Addendum: there have been many references to the Wikipedia page on Schwarzschild's equation. One of the references it uses is a very good post over at Science of Doom. That post has lots of graphics and explanations of what "climate science" actually does (which is quite different from CallItAsItIs's fictional parallel universe version). It also provides comparisons between theoretical calculations and measurements. At one point, that post states:
When CallItAsItIs gets his poetry published in a respected scientific journal, it might be worth taking another look.
Further addendum: From the Conclusion of the Science of Doom page:
This is getting really goofy. Radiative transfer has been worked out by very qualified people and has produced very precise models that have been extensively validated by measurements. These results allow for IR weapon guidance from sea level through 60,000 ft and even higher, have countless other scientific and engineering applications. That ship has sailed and is navigating commercially. I see someone arguing that a wheel can't work because there is no relative motion between the wheel and the ground where they contact, then trying to reinvent the wheel, and having no success whatsoever. It has become tedious and painful to watch.
My apologies, Moderator, for my clumsy communication. The aim was to point out the colossal temporal difference in orders of magnitude between the molecular/photonic interplays versus the meteorological layers of the atmosphere . . . was a difference so vast that it left no room for prevarications by poster CallItAsItIs about "equilibrium" being present or not present.
Far more delightful was Philippe's [@865] metaphor about the "stationary" wheel rolling down the road. ( Just so long as Philippe avoids mentioning that smoky second of impact when the landing wheels touch down on the runway! )
No problem. The moderator comment was intended to put some additional numbers on the picture.
The 10us for relaxation time can be used to estimate the total temperature change that occurs in the time scale of reference. For example, at a rate of 0.2C/decade for global warming, we would expect that to contribute 0.02C/year, or 0.000055C/day, or 6.3x10-10C per second, or 6.3x10-13C/millisecond, or 6.3x10-15C in 10 us.
But apparently there are people in this world that think that a changing climate with a temperature change of 6.3x10-15C is not close enough to zero.
It is not pragmatic to be measuring with a micrometer, marking with chalk, and cutting with an axe.
I should perhaps correct the typo @864.
Schwarzschild's equation is expressed correctly thus:-
dIλ/ds = nσλ[Bλ(t)-Iλ]
where
Bλ(t) = [(2h.c^2)/λ^5] x 1/[e^(hc/kBt )-1]
This looks eminently solvable (said boldly he) as it does appear to convert into a simple series for a linear-changing temperature. But there really is no reason to do this solving as through a cooling temperature gradient the equasion shows the flux will be dropping and ever approaching Iλ = Bλ(t). And this will be the case up through the troposphere until either the process continues up past the tropopause or the process ends with emissions from within the tropopause out into space.
And perhaps I should also explain the "???" @549 where I claim "As a PhD-wielding engineer, this extra source ???→CO2→photon comes as no surprise to me."
Actually an undergraduate engineer should be able to provide such an explanation if they had been paying attention during the lectures on Specific Heat Content. These show a lot more going on with poly-atomic gases than be explained by Ideal Gas theory and that activity is the spinning and vibrating of the molecules. And it is, of course, vibrating molecules that emit IR in the atmosphere.
Greetings SkS folks,
I am an associate of CallItAsItIs and would like to inform you of the completion of his paper titled Sensitivity Assessment of the C 2 Greenhouse Effect which specifically addresses MA Rodger@864 who states
Whatever the derivation of Schwarzschild's equation, we expect you to use it to demonstrate your proposed IR extinction phenomenon mathematically. I do not see where Kirchoff's Law would be required for such a mathematiacl [mathematical] exercise.
Regarding Kirchhoff's Law, since it was used in developing the Schwartzschild equation, it is "automatically" used when solving this equation. It is not somehow implemented in another step. So, it seems that moderator Response needs to update the statement that
... Your tireless, empty assertions of having an alternate explanation for radiative transfer equations will only be believed if you actually present such an explanation, in full. (Emphasis original)
The only conclusion that we can draw at this point is that you actually cannot provide such an explanation. (Emphasis added)
Not only that, but this moderator knew full well that "such an explanation, in full" was not possible for CallItAsItIs due to the fact that equations were essential to his arguments, and this website does not support posting equations on the Post-a-Comment pages. Instead of getting help in this matter, however, he got comments such as "more excuses to not do your own work", and his logins were disabled over a few days. Can we say just plain childish!? But perhaps on a more interesting note is the comment
... Your tireless, empty assertions of having an alternate explanation for radiative transfer equations ... (emphasis added)
Just wait until this moderator learns that the Beer-Lambert and Schwartzschild equations are radiative transfer equations (LOL!), but I suppose that's typical of the understanding this individual has about the actual science. It seems that he/she can "snip", but that's about it!
Anyway, in addition to solving the Schwartzchild equation, CallItAsItIs shows mistakes and mis-understandings on both sides of this issue, and resolves some seeming conflicts between the two. It turns out that the greenhouse forcings (ie. the heat energy per unit area per unit time) obtained from the Schwartzschild solutions were identical to those he predicted from the Beer-Lambert equation with no thermal emissions and no regard for thermal equilibrium. Actually, this should not be too surprising since absorption depends directly only the the current intensity, absorption cross-section, and CO2 density — and not thermal equilibrium status. While the absorption cross-section and CO2 density may in turn depend on temperature and pressure, it is assumed here that these profiles are known and part of the "given" for this exercise.
Now, the original solution with the Beer-Lambert equation does have the limitation of not being able to predict the 15 micron thermal emissions emanating from the TOA, but these can be calculated separately using the Planck thermal distribution and subtracted from the total band intensity recorded by the detectors. It is important to realize that these thermal emissions consist only of photons escaping from the atmosphere and therefore cause no warming within the atmosphere. Hence, the only difference between these solutions is that in one case, the greenhouse effect forcing is determined in one calculation and the atmospheric thermal emissions flux is obtained in a separate step; and in the other case, both of these values are obtained from the solution of one somewhat more complicated equation. In either case, both bottom-line values are identical.
So, if you would like a copy of this paper please send me an email to answers@sciencefortruth.com and I will be glad to send you a copy in the .pdf format. Rob Loblaw does have a valid point when he says "do the actual math", and CallItAsItIs has done just that. In posting this message, I am hoping (against hope!) that your moderator would see this as a possible breakthrough in our understanding of theories that are in fact generally accepted in the scientific community, including climate scientists. But I'm not counting on it. So if you are interested, I would suggest that you act quickly in getting your email message out to me.
Contents deleted. Sock puppet banned.
Now I'm just an amateur at this, but I believe I can quickly resolve this CO2 band saturation issue without getting into real complicated discussions. First, we note that for an unsaturated atmosphere, we expect the upward 15 micron radiation to monotonically decrease with altitude until approaching the TOA. This is because the CO2 molecules continue to tap energy from this upward IR radiation for as long as they are there to do so, thereby depleting energy from the 15 micron band and causing a drop in band intensity.
So, is this what is happening in the case of the 15 micron CO2 absorption band? Well, let's see! The MODTRAN plots supplied by Bob Loblaw@731 will be most helpful. Here, he has plotted spectral intensity vs wavelength of upward terrestrial IR radiation at the altitudes of 10km, 20km, 30km, 50km, and 70km. Examining the values of the spectral intensity at 15 microns I15(z) in each of these plots, we see that while there was a drop in I15 in going from z=10km to z=20km, there was a definite increase in going from 20-30km, and again in going from 30-50km. There was not much of a change in I15, however, in going from 50-70km. Therefore, it appears that in Bob Loblaw's example, the upward-bound 15 micron radiation intensity approaches a constant value well below the TOA while in an unsaturated atmosphere, this upward-bound radiation intensity monotonically decreases as altitude increases until it approaches the TOA.
So, is the CO2 greenhouse effect saturated of unsaturated? You tell me!
No, you're another sock puppet. You are wasting everyone's time - especially yours.
[addendum]
For anyone puzzled by this reaction, read definition number 3 here:
..and then review the Comments Policy, where it states:
As part of my New Year's Resolutions, I had wished to ask if SkS readers have any views or opinions on the AGW/CO2 ideas put forward by a Mr (or Dr ?) Tom Shula who allegedly has some physicist qualifications, yet who also qualifies as a science-denier apparently.
He strongly asserts that the 15-micron IR radiated upwards by the Earth's warm surface is totally absorbed by CO2 in the lowermost 10 meters of the troposphere. And that there is no re-radiation of IR by the CO2 molecules in that 10-meter layer ~ because, although molecules in solids and liquids can radiate photons, nevertheless it is impossible for molecules in the gasseous state to emit radiation.
At the same time, Tom Shula does say that Earth emits IR to space from the upper troposphere. Clearly Tom Shula suffers from cognitive dissonance . . . or I am failing to grasp the subtleties of his ideas.
IIRC, someone earlier in this thread has also claimed that "CO2 saturation" means that the 15-micron IR from the Earth's surface cannot rise above the 10-meter altitude (possibly that claimant was poster CallItAsItIs or his re-incarnations ~ but he has made so many posts in this thread, that it would be a tiresomely daunting task for me to go back and check & read through all of his comments ).
.
The assertions by Shula were made in a Youtube video in 2024, on the Tom Nelson channel ~ a channel which is often bristling with Red Flags of Unscience.
Any thoughts? BTW, I am quite happy if this post today elicits no replies at all . . . for it is highly probable that Tom Shula's (and Tom Nelson's ) effusions are a waste of time for rational readers.
[BL] This sounds waaay too much like the arguments being made many times here over the years by a series of banned users - most recently calling himself CallItAsItIs (then ReadItAndWeep, and finally LinusLucy).
The user that has made multiple identities here is clearly immune to logic and reason, and there is no point in discussing his hallucinations again.
[addendum]
After looking a bit more at what Tom Shula has been presenting, it seems unlikely that Tom Shula has been the person posting here. Consider them to be equally-deluded and sharing misinformation space, but probably not the same person.
[BL] @870 ~ thanks for the Addendum. My impression is likewise, that Tom Shula is unlikely to be poster CallItAsItIs et al., despite both having similar delusions.
The "CO2 is Saturated" argument depends on the concept of non-radiation of photons by GreenHouse gasses in the lowermost 10 meters of the troposphere . . . and yet Tom Shula admits that the same gasseous molecules can radiate at the uppermost level of troposphere. Go figure!
Digging shows that Shula did make an OP at WUWT in 2023, but his Youtube presentations have scored no more than 19,000 views to date (earliest was 4 years ago). #They are lengthy and complex, and largely correct apart from a few blatant misrepresentations.
His most egregious misrepresentation was the assertion that, once a molecule is in a gas, it is unable to emit photons. A truly remarkable assertion ~ considering that after sunrise every day, Tom Shula's own eyes can see the photons radiated by the gasseous molecules of the Sun.
Eclectic @870/871,
Thomas Shula is a retired broker who did physics back in his university days.
As for the video, your "Youtube video in 2024, on the Tom Nelson channel" seems to be to a different to the one HERE (it stretches to almost 2 hours) which is basically a presentationt of a 26-page thesis posted HERE July 2024 and entitled 'The “Missing Link” in the Greenhouse Effect'.
The work is only co-authored by Shula along with one Dr Markus Ott, a german chemist who previously authored a whole book on the subject entitled 'Dismantling The CO2-Hoax'.
I've only skimmed through this Shula/Ott stuff (so far) but note two rather odd-but-fundamental lapses of logic by these two gentlemen.
Their first lapse (which is very badly misrepresented by Shula in the video @0:3:23 when he boldly goes off script and states that emitting IR "is a property of condensed matter. Gases do not emit thermal radiation."): this first lapse is to agree that almost all IR absorbed by CO2 is 'thermalised' (because the average relaxation time required to emit IR is measured in tenths of a second while the disrupting impacts of fellow air molecules occur on average in microseconds) but then they entirely ignore the effect of these far-more-numerous molecular collisions causing the vast majority of CO2 population which is in the excited state and thus can (and so many of them that it does) emit the IR.
And the existence of such radiation is readily measured as the back-radiation at grond level. So it is a pretty silly error.
The second lapse is to fail to grasp that their magic "missing link" would still work to give us AGW.
Their "missing link" is to suggest that, with the absorbed IR at 15-microns entirely 'thermalised' in the thick air at low altitude and in their version not re-emitted as IR, the energy in this 15-micron waveband is transmitted up to an altitude by means of convection, up to thinner air where (acording to them) 'thermalisation' is weakened enough to allow emitted IR. And at such altitudes the IR is emitted out into space. In my quick skim-through I've not spotted any reason why their replacing the 15-micron IR flux up through the atmosphere with their "missing" convection flux would mpact the level of TOA emissions and how, with an increasing altitude for such emissions with increasing CO2, why the flux wouldn't result in AGW (as it does).
MA Rodger @872 :-
Please don't bother to spend more of your own valuable time going through the Shula videos !
As you mentioned, they are very lengthy ~ much of what he says is paleo information which is not in dispute. Possibly that lengthy info is intended to camouflage the actual unscientific "clangers" which he comes out with, scattered here & there in his videos.
Also as you say ~ in that one video, in less than 5 minutes, he states boldly that "Gases do not emit thermal radiation". And I persisted for about one-third of the total video, in the hope that he was really going to quibble about the semantics of "thermal" . . . but it was not to be, for he was simply flat wrong in his understandings of the science.
Other red flags were Shula's use of a rather shonky Scotese paleo graph . . . and later Shula's use of a Holocene graph of global CO2 levels versus a temperature graph (without pointing out that the temperatures were Greenlandic not global) ~ from both graphs he casually asserted that there has been zero evidence of CO2/temperature linkage.
So, the Shula case is quite hopeless . . . but these modern videos are still coming out, and are misleading the unwary public (such as poster CallItAsItIs).
Viewing smaller amounts of other Shula ( +/_ colleague named Ott ) videos has not given any other new insights into the deniosphere claims that "CO2 effect is saturated".
Schroeder/CallItAsItIs @874
You are fooling no-one here. How can the Beer and Schwartzschild solutions be the same? Beer concerns only absorption and Schwartzschild considers both absorption and emission. If they were the same, it could only be through emission being zero, but that is not the case.
dIλ/ds = -μIλ ≠ dIλ/ds = nσλ[Bλ(t)-Iλ]
excepting Bλ(t) = 0
And it is because of this emission from CO2 that we see 15-micron IR upward (and downward) throughout the atmosphere. It is not "attenuated to near zero at an altitude of 10 meters." And your attempt to explain the existence of 15-micron IR emissiions from CO2 are nothing but garbled nonsense. Consider the MODRTAN model (which you appear to accept but perhaps don't understand), the IR emissions for a doubling of CO2 from the default values (400ppm to 800ppm) are calculated as falling from 298.52Wm^-2 to 295.191Wm^-2, a global forcing of +3.3Wm^-2. So MODTRAN is showing that the CO2 effect is not saturated.
As yet another account created in violation of the Comments Policy, the user Schroeder that you are responding to has had all comments deleted and the account banned.
In order for emission to be zero, you need at least one of two things:
All the ballyhoo about "thermal radiation" represents a special pleading that the IR radiation in question does not follow the laws of radiation transfer that all other forms of radiation follow.
Schroeder/CallItAsItIs/et alia @874 :-
You are in danger of sounding like the hallucinational garbled nonsense produced by an un-monitored Artificial Intelligence.
Stop. Reflect. Think about the physical situation of a nitrogen/oxygen atmosphere with CO2 and H2O molecules dispersed throughout. If (as is indeed the case) these CO2 & H2O molecules at altitude 50 meters or 1500 meters get "thermally stimulated" by other neighbouring air molecules, then some of those CO2 or H2O molecules will emit IR photons (as is indeed observed and measured).
And that IR emission activity (throughout the troposphere) means that your whole line of argument falls flat on its face.
So please ignore the rubbish coming from Tom Shula.
It is most unfortunate that the user most recently using the Schroeder moniker keeps wasting time by creating new accounts, instead of using his time to good stead by actually learning some physics.
There is a parallel between him and Shula in that they both seem to think that emission of IR does not happen in the atmosphere until you get close to the TOA. It has been repeatedly pointed out that their "non-existent" IR radiation has been measured, but that never seems to stop them from trying to convince people otherwise.
Quoted in John Grant's Denying Science: