Is the CO2 effect saturated?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
The notion that the CO2 effect is 'saturated' is based on a misunderstanding of how the greenhouse effect works. |
|||||||
Climate Myth...
CO2 effect is saturated
"Each unit of CO2 you put into the atmosphere has less and less of a warming impact. Once the atmosphere reaches a saturation point, additional input of CO2 will not really have any major impact. It's like putting insulation in your attic. They give a recommended amount and after that you can stack the insulation up to the roof and it's going to have no impact." (Marc Morano, as quoted by Steve Eliot)
At-a-Glance
This myth relies on the use (or in fact misuse) of a particular word – 'saturated'. When someone comes in from a prolonged downpour, they may well exclaim that they are saturated. They cannot imagine being any wetter. That's casual usage, though.
In science, 'saturated' is a strictly-defined term. For example, in a saturated salt solution, no more salt will dissolve, period. But what's that got to do with heat transfer in Earth's atmosphere? Let's take a look.
Heat-trapping by CO2 in the atmosphere happens because it has the ability to absorb and pass on infra-red radiation – it is a 'greenhouse gas'. Infra-red is just one part of the electromagnetic spectrum, divided by physicists into a series of bands. From the low-frequency end of the spectrum upwards, the bands are as follows: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Gamma rays thus have a very high-frequency. They are the highest-energy form of radiation.
As our understanding of the electromagnetic spectrum developed, it was realised that the radiation consists of particles called 'photons', travelling in waves. The term was coined in 1926 by the celebrated physicist Gilbert Lewis (1875-1946). A photon's energy is related to its wavelength. The shorter the wavelength, the higher the energy, so that the very high-energy gamma-rays have the shortest wavelength of the lot.
Sunshine consists mostly of ultraviolet, visible light and infra-red photons. Objects warmed by the sun then re-emit energy photons at infra-red wavelengths. Like other greenhouse gases, CO2 has the ability to absorb infra-red photons. But CO2 is unlike a mop, which has to be wrung out regularly in order for it to continue working. CO2 molecules do not get filled up with infra-red photons. Not only do they emit their own infra-red photons, but also they are constantly colliding with neighbouring molecules in the air. The constant collisions are important. Every time they happen, energy is shared out between the colliding molecules.
Through those emissions and collisions, CO2 molecules constantly warm their surroundings. This goes on all the time and at all levels in the atmosphere. You cannot say, “CO2 is saturated because the surface-emitted IR is rapidly absorbed”, because you need to take into account the whole atmosphere and its constant, ongoing energy-exchange processes. That means taking into account all absorption, all re-emission, all collisions, all heating and cooling and all eventual loss to space, at all levels.
If the amount of radiation lost to space is equal to the amount coming in from the Sun, Earth is said to be in energy balance. But if the strength of the greenhouse effect is increased, the amount of energy escaping falls behind the amount that is incoming. Earth is then said to be in an energy imbalance and the climate heats up. Double the CO2 concentration and you get a few degrees of warming: double it again and you get a few more and on and on it goes. There is no room for complacency here. By the time just one doubling has occurred, the planet would already be unrecognisable. The insulation analogy in the myth is misleading because it over-simplifies what happens in the atmosphere.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
This myth relies on the use of a word – saturated. When we think of saturated in everyday use, the term 'soggy' comes to mind. This is a good example of a word that has one meaning in common parlance but another very specific one when thinking about atmospheric physics. Other such words come to mind too. Absorb and emit are two good examples relevant to this topic and we’ll discuss how they relate to atmospheric processes below.
First things first. The effect of CO2 in the atmosphere is due to its influence on the transport of 'electromagnetic radiation' (EMR). EMR is energy that is moving as x-rays, ultraviolet (UV) light, visible light, infrared (IR) radiation and so on (fig. 1). Radiation is unusual in the sense that it contains energy but it is also always moving, at the speed of light, so it is also a form of transport. Radiation is also unusual in that it has properties of particles but also travels with the properties of waves, so we talk about its wavelength.
The particles making up radiation are known as photons. Each photon contains a specific amount of energy, and that is related to its wavelength. High energy photons have short wavelengths, and low energy photons have longer wavelengths. In climate, we are interested in two main radiation categories - firstly the visible light plus UV and minor IR that together make up sunshine, and secondly the IR from the earth-atmosphere system.
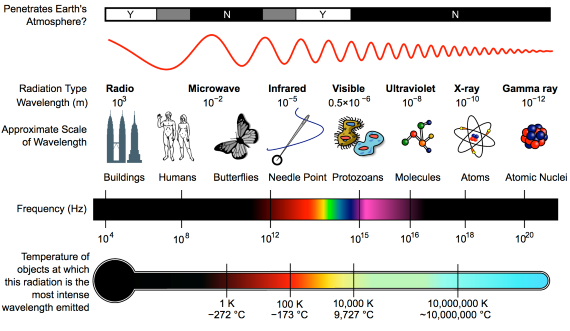
Fig. 1: diagram showing the full electromagnetic spectrum and its properties of the different bands. Image: CC BY-SA 3.0 from Wikimedia.
CO2 has the ability to absorb IR photons – it is a 'greenhouse gas'.So what does “absorb” mean, when talking about radiation? We are all familiar with using a sponge to mop up a water spill. The sponge will only absorb so much and will not absorb any more unless it's wrung out. In everyday language it may be described, without measurements, as 'saturated'. In this household example, 'absorb' basically means 'soak up' and 'saturated' simply means 'full to capacity'. Scientific terms are, in contrast, strictly defined.
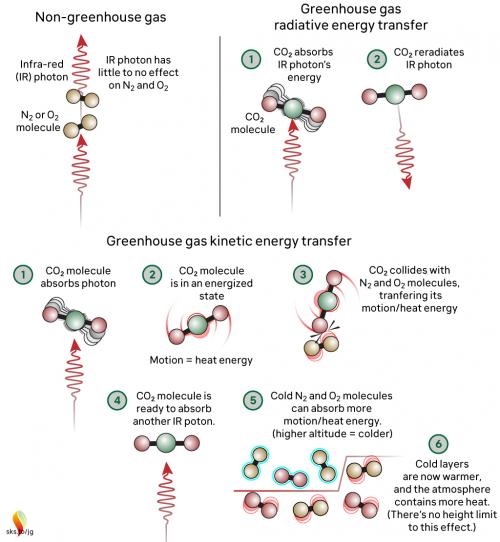
Now let's look at the atmosphere. The greenhouse effect works like this: energy arrives from the sun in the form of visible light and ultraviolet radiation. A proportion reaches and warms Earth's surface. Earth then emits the energy in the form of photons of IR radiation.
Greenhouse gases in the atmosphere, such as CO2 molecules, absorb some of this IR radiation, then re-emit it in all directions - including back to Earth's surface. The CO2 molecule does not fill up with IR photons, running out of space for any more. Instead, the CO2 molecule absorbs the energy from the IR photon and the photon ceases to be. The CO2 molecule now contains more energy, but that is transient since the molecule emits its own IR photons. Not only that: it's constantly colliding with other molecules such as N2 and O2 in the surrounding air. In those collisions, that excess energy is shared with them. This energy-sharing causes the nearby air to heat up (fig. 2).
Fig. 2: The greenhouse effect in action, showing the interactions between molecules. The interactions happen at all levels of the atmosphere and are constantly ongoing. Graphic: jg.
The capacity for CO2 to absorb photons is almost limitless. The CO2 molecule can also receive energy from collisions with other molecules, and it can lose energy by emitting IR radiation. When a photon is emitted, we’re not bringing a photon out of storage - we are bringing energy out of storage and turning it into a photon, travelling away at the speed of light. So CO2 is constantly absorbing IR radiation, constantly emitting IR radiation and constantly sharing energy with the surrounding air molecules. To understand the role of CO2, we need to consider all these forms of energy storage and transport.
So, where does 'saturation' get used in climate change contrarianism? The most common way they try to frame things is to claim that IR emitted from the surface, in the wavelengths where CO2 absorbs, is all absorbed fairly close to the surface. Therefore, the story continues, adding more CO2 can’t make any more difference. This is inaccurate through omission, because either innocently or deliberately, it ignores the rest of the picture, where energy is constantly being exchanged with other molecules by collisions and CO2 is constantly emitting IR radiation. This means that there is always IR radiation being emitted upwards by CO2 at all levels in the atmosphere. It might not have originated from the surface, but IR radiation is still present in the wavelengths that CO2 absorbs and emits. When emitted in the upper atmosphere, it can and will be lost to space.
When you include all the energy transfers related to the CO2 absorption of IR radiation – the transfer to other molecules, the emission, and both the upward and downward energy fluxes at all altitudes - then we find that adding CO2 to our current atmosphere acts to inhibit the transfer of radiative energy throughout that atmosphere and, ultimately, into space. This will lead to additional warming until the amount of energy being lost to space matches what is being received. This is precisely what is happening.
The myth reproduced at the top – incorrectly stating an analogy with roof insulation in that each unit has less of an effect - is misleading. Doubling CO2 from 280 ppm to 560 ppm will cause a few degrees of warming. Doubling again (560 to 1130 ppm) will cause a similar amount of additional warming, and so on. Many doublings later there may be a point where adding more CO2 has little effect, but recent work has cast serious doubt on that (He et al. 2023). But we are a long, long way from reaching that point and in any case we do not want to go anywhere near it! One doubling will be serious enough.
Finally, directly observing the specific, global radiative forcing caused by well-mixed greenhouse gases has - to date - proven elusive. This is because of irregular, uncalibrated or limited areal measurements. But very recently, results have been published regarding the deep reinterrogation of years of data (2003-2021) from the Atmospheric Infrared Sounder (AIRS) instrument on NASA's Aqua Satellite (Raghuraman et al. 2023). The work may well have finally cracked the long-standing issue of how to make finely detailed, consistent wavelength-specific measurements of outgoing long-wave radiation from Earth into space. As such, it has opened the way to direct monitoring of the radiative impact (i.e. forcing + feedback) of greenhouse gas concentration changes, thereby complimenting the Keeling Curve - the longstanding dataset of measured CO2 concentrations, down at the planet's surface.
Note: Several people in addition to John Mason were involved with updating this basic level rebuttal, namely Bob Loblaw, Ken Rice and John Garrett (jg).
Last updated on 31 December 2023 by John Mason. View Archives































 Arguments
Arguments





































Oh, what a complex subject radiation transfer is...
First, I have not watched Jonathan's video. At 30 minutes long, a video is a very slow way of gaining an understanding. I'd rather read.
There is a similarity between atmospheric transfer of IR radiation and headlights in fog, but there are also significant differences.
First of all, photons travel in fairly straight lines. (We'll ignore refraction for now.) Photons passing through a medium (e.g. the atmosphere) can basically do three things:
IR doesn't get scattered in the atmosphere - the wavelengths and molecular and particle sizes don't match up. It either is transmitted or absorbed. The ratio is expressed by Beer's Law, which is logarithmic. Over a given distance, if 10% is absorbed and 90% transmitted, then the next equal distance will absorb another 10% - but of the 90% that was transmitted - i.e., 0.1*0.9 = 9% of the original, not 10% of the original.
The transmitted amount after 2 units is 0.9*0.9 or 81%. Over N units of atmosphere, the transmitted amount passing through is 0.9^N - so after 10 units of "10% per unit" we get 35% transmission, not 0% (10*10%). At 50 units, we're now at 0.5% transmission.
Is the transmission "saturated" at 50 units? Some might say yes, because if we increase the absorption per unit to 15% (thus, transmission per unit = 85%, or 0.85), then at 50 units we see 0.03% transmission (0.85^50), but so what? We only had 0.5% before anyway... is there really any difference between 0.5% and 0.03%? If we look at what is going on at 10 units, however, 0.85^10 = 0.20 or 20%. At 10 units, we've dropped from 34% transmission to 20%. The concept of "saturation" is now dependent on where we are. What seemed "saturated" at 50 units doesn't quite look that way at 10 units.
In the case of transmission of IR from the surface to the top of the atmosphere, even if (at some wavelength where CO2 absorbs) there is next to no radiation reaching the top of atmosphere directly, adding CO2 can still affect how much reaches a lower altitude directly - i.e., the average distance travelled before absorption will decrease. Although almost nothing reaches 100km directly, the amount reaching 10 km may now only reach 9km, or 8km, or 7km...
Once absorbed, energy will be re-radiated as IR again, but the re-radiation happens in all directions. Half of that will be moving down, not up, and to get it out to space, it will have to be re-radiated several times. The more CO2, the more times it will be absorbed and re-radiated, and the more times that happens the less efficient it is and the warmer the surface must be. This is the basic greenhouse effect.
Now, back to lights in fog. Most of the light passing through fog will be either transmitted or scattered - not much is absorbed. The amount transmitted directly still follows Beer's Law, though. A lot of the scattered light still travels forward, but we'll be seeing it from other angles - not directly. We can "see" the lights as long as the amount of light transmitted directly is still greater than the amout we see that is being scattered by the fog. Once too much of the direct light is scattered, the headlights get "lost in the fog".
When we can't see the lights at 100m, we might say "the fog is saturated", but at 50m we can still see the lights. (Tom makes this point above.) If the fog gets heavier, the person at 100m can't tell the difference, but the person at 50m does - until she can't see the lights. Another person at 25m still does, though, and when the fog gets heavier and you can't see the lights at 25m, you still can at 12m, etc., etc. etc. Each observer is looking at exactly the same lights and atmosphere, so if only some of the observers are saying "it's saturated" and other aren't, then the concept of "saturation" is not actually a universal property of either the lights or the fog - it is something that tells us more about the observer's position than anything else. This is not a useful concept for radiation transfer.
Now, to compare IR to lights in fog, the big difference is absorption vs. scattering. But just as absorbed IR is re-radiated both up and down, the lights are scattered both forward and backwards. The observer standing 50m from the car will see the lights fade into the fog as it gets heavier, and the driver of the car will get more of the light shining back in their face as the fog gets heavier. What the driver sees in back-scatter is similar to IR "back-radiation". Even though the observer at 100m says "the fog is saturated", the driver can tell the fog is getting heavier. Just as the case with increasing atmospheric CO2: although the observer at the top of the atmosphere might be thinking "it's saturated", the surface can tell that the IR effect is increasing.
In another simliarity, although the viewer of the car can't see headlights at 100m, they do see light (let's do the thought experiment on a dark road at night). Brighter headlights won't help the viewer see the headlights, but the fog-light will increase. Just the same, as the earth's surface temperature increases, you still won't see direct IR loss to space, but the total IR transfer upwards can increase.
KR @298:
Where do you get the "absorption of IR is effectively saturated near the ground at sea-level pressures, with the average absorption path length being quite short (in the order of meters)" statement from? Even at wavelengths were CO2 absorbs, my impression is that it can travel significant distances. IR spectromoetry is a common method of measuring atmospheric CO2 concentrations, and commerical sensors can handle up to 3000ppm, IIRC, suggesting that at 300-400ppm we're far short of "saturation".
Bob Loblaw - Sorry if I wasn't specific enough, that's absorption at major GHG frequencies. There are plenty of atmospheric windows and lower sidebars of partial transmission, but for strongly absorbing frequencies CO2 has a mean sea level path length of ~33 meters or so.
IR gas analyzers, on the other hand, are generally less than a meter in any dimension :)
Ach, horrible reference in my last message - turns out the author is a denier of CO2 as a GHG, my apologies. He doesn't seem to understand what happens when there is a change in effective radiating altitude. Still, the path length discussion is reasonable.
Bob... Thanks. That makes sense to me. I figured that Jonathan had essentially tripped himself up with his insistance on using the fog metaphor. He makes that error very early in the video and essentially continues off in that direction for the rest of the 20+ minutes.
KR... Yup. He's a CO2 as a GHG denier, and a college professor as well.
KR:
You prompted me to dig out my copy of Pierrehumbert's Principles of Planetary Climate, but unfortunately his graphs of CO2 absorption coefficients are in units of m2/kg and there is no easy conversion to simple units of length. Too much math for an evening at home at the end of a long weekend.
...but, to read the reference you linked to, what I find there is a series of calculations ultimately based on density, which appear to estimate how much of the volume of air is occupied by CO2. This is used to calculate the "mean free path length of quantum/waves in the atmosphere before colliding with a molecule of carbon dioxide".
What I do not see there is any discussion of how often a "collision" [and I use that term loosely] actually leads to absorption. Nor do I see any indication that the calculation takes wavelength into acount, which is a huge factor in absorption. (CO2 only absorbs at selected wavelengths.)
I suspect, but have not done the math to confirm, that if you repeated the calculation for nitrogen (much more prevalent in the atmosphere) that you'd get an even shorter "mean free path length" for N2 - but we know that N2 does not absorb IR strongly. He does seem to think that "molecules of water vapor and solid particles" in the atmosphere are much more important, basically because photons will run into them first.
In other words, I think the 33m that he comes up with has absolutely nothing to do with how far a typical IR photon travels in the atmosphere before being absorbed by CO2. We do know that photons outside the CO2 absorption wavelengths will go a lot further than ones at the appropriate CO2 wavelengths, and his calculations do not identify that.
Oddly, even though he calculates a "mean free path length" much shorter than I would expect for the distance before IR absorption, he also uses this to conclude that CO2 is not a greenhouse gas. This conclusion appears to be due to the speed with which an IR photon can escape the atmosphere.
Overall, I think his math is just a bunch of technobabble that amounts to an argument that trace gases can't be radiatively active. It's an odd flavour of CO2 is just a trace gas.
Rob:
Not just a Professor, but a "Scientific Research Director". Unfortunately, if I try a Google search for "Biology Cabinet Division Mexico" - his stated location. I only seem to find links to other copies of his paper at the usual denial web sites. He's a legend in his own mind.
KR:
I did find a set of lecture notes at this link. Figure 7.3 seems to suggest that even at the peak absorption bands you see a few percent of IR reaching 13km altitude from the surface, but that graph is at higher wave numbers (lower wavelengths) than the most active IR absoprtion bands. Figure 7.2 is a much lower resolution, but does show the greatly-increased absorption by CO2 as you move out into the longer wavelengths.
Bob
Interesting reference. Unfortunately the graphs cover different parts of the spectrum.
Fig 1 is for CO2, smack bang in the center of its absorption curve. And it shows just how big a factor line-broadening is.
Also it answers the important question of absorption path length, at least near the peak. The upper graph in Fig 1 is essentially sea level pressure. Right at the peak at 667.5 cm-1 there is only about 3% transmission - 97% absorption - after 1 meters. After even 10 meters that would be 0.0310 or 0.000000000000059049%
Even just at 667.0cm-1 there is about 83% transmission - 17% absorption - after 1 meters. So about 15.5% transmitted after 10 meters and it takes around 170 meters to match the absorption at the peak.
Fig 2 actually cuts off the peak absorption region for CO2 on the far right just as it has dropped to maximum absorption.
Fig 3 is centered near the peak of the Ozone absorption, with only a modest role for CO2 there.
Referring to the opening equation - 7.1 - the Spectral Intensity S of CO2 is one of the key factors here. The other factor, the function f() covers line broadening etc and is where a lot of the complexities are.
This is a graph of the value of S for CO2 over much of its key absorption band, taken from the HITRAN database using the website spectralcalc.com
Notice the vertical scale is logarithmic. There is a roughly 6 orders of magnitude difference between the height of the peaks at the center and the peaks at the edges. Those are the wings.
Glenn Tamblyn - Far more accurate than the references I was looking at.
"Saturation" is a horrible term, really, inappropriate for an exponential decrease with distance as there is no specification of an end-point. Far better to use e-fold distance or mean path length - some measure with actual values.
So absorption path lengths for GHGs can be quite short at sea level depending on wavelength. The biggest issues with the "CO2 is saturated" claims are that:
Tom Curtis @295
Thanks.
Here is a video to give some more detail.
LINK
I think pedagogically I'm going to tend toward the use of k = T*1.96/(cm•K) for my Wein's Law needs. Though lambda • T = .0029 meter Kelvin will come in handy sometimes, too.
To Rob @299 and KR @300
I'm still looking into this issue. How is the analogy "apt" and how is it not?
The headlights should not only illuminate the fog, but should actually bounce off the fog. When the light bounces off the fog, since light has momentum, it does change the kinetic energy of the molecules in the fog.
But when you turn the headlights off, the fog does not continue to glow in visible light. Rather, the light dissipates fairly quickly.
I guess I have two major questions in this area. (1) Why does the visible light reflect off the fog, when it does not reflect off a region of no fog? My hypothesis would be that H20 has a significant absorption/emission spectrum in the visible range, wheras Oxygen/Nitrogen/Argon do not.
(2) Why does the light immediately dissipate once the headlights are turned off? My hypothesis would be that the internal energy of the fog is not sufficient to activate those modes of motion.
In any case, to feel complete to me, a model must include reflection, and transparency, (and possibly refraction) as well as absorption and emission...
Checking my assumptions, I'm thinking that a gas cannot emit, absorb, or reflect light in any spectrum where it is completely transparent. But fog is not transparent in visible light, so by that assumption must be absorbing, emitting, and reflecting visible light. It's just not heating to the point where the visible light becomes a significant part of its blackbody spectrum.
Jonathan Doolin - Major issues: scattering is not absorption.
Scattering redirects incoming light in an anisotropic (angle-dependent) fashion. Fog scatters visibile light with neglible absorption, and when the headlights turn off the scattered light ceases as well. Think pachinko balls for this. There is a very small amount of momentum transfer involved, nothing on the level of thermal absorption.
Absorption raises the energy of the absorbing molecule, contributing to the thermal energy of the gas - if all other things are held equal an equivalent amount of past absorbed energy spread across the emission spectra will be thermally emitted by that gas volume at some later point (not, generally, at identical frequencies to incoming photons, or from the same molecules, but in the same spectra range), isotropically. This is a completely different phenomena.
So no, fog is not an "apt" analogy to absorption/emission in any way. If you start from that as your analogy you are already pointing in the wrong directions, even without the logical error of trying to reason analogically rather than in the system of interest. "If you think that the line of argument that led you to believe something about X might also apply to Y, don't talk about X. Apply the reasoning to Y and see if it works."
Jonathan Doolin - I took a quick look at your video, and there are numerous conceptual issues present.
A 'blackbody' emits as per the dotted lines shown for various temperatures. Perfect blackbodies are not real, although some objects approach it for specific frequency bands. The ground, for example, has an emissivity of 98-99% in the IR range (98-99% of an ideal blackbody) - anything less than that in satellite observations indicates emission from something else in the atmosphere, rather than in an IR transparent window.
What you are looking at in any of the satellite or ground spectra is the result of many different molecules, many different gases and/or materials. CO2, H2O, O3, and other gases absorb and emit based on their molecular structure, acting as frequency dependent antennae. And the different levels in the spectra reflect what temperature the emitting object is, and (by the lapse rate) where it's located in the atmospheric column. You cannot treat this as the emission from a single object or material.
The small graph you looked at for an ozone peak appears to be from Harries 2001, and is a graph of changes in emission to space from 1970 to 1996 with increasing GHGs - it's a delta graph.
---
I would strongly suggest some introductory reading to orient yourself to the general principles involved, such as the Greenhouse Effect Basics on SkS. To be quite frank, the gaps in your understanding will otherwise will prevent any useful technical reasoning on the issues.
Jonathon
I agree with KR that using analogies to understand something can be problematic. And your introduction of 'reflection' and 'diffraction' is inaccurate. Reflection only occurs when light interacts with a surface of a solid or a liquid. It does not apply to a gas. Similarly diffraction occcurs when light passes through a surface into a solid or liquid.
In the case of fog both of these things occur. Light is reflected off the surface of the drops of water and is also diffracted through the drops. Additionally there is also scattering of the light by the droplet. In all three cases the photon of light is not extinguished, its trajectory is simply changed.
None of these three phenomena are relevent to understanding what is happening wrt the GH gases. Reflection and diffraction don't apply and at the infre-red wavelengths involved here scattering is negligible.
So everything wrt GH gases is about absorption and emission. In absorption the GH molecule actually absorbs the photon. The energy of the photon is added to the energy of the molecule and the photon ceases to exist. In emission the molecule actually creates a photon which 'launches' away in a random direction, taking some of the molecules energy with it, leaving the molecule with less energy.
The form the energy takes within the molecule is important. A molecule can be thought of as a collection of atoms that are joined by elecrical bonds. The bonds allow the individual atoms to jiggle around relative to each other - the molecule isn't a rigid body. You could think of it as if each atom in the molecule is on springs connecting it to its partners. So there is energy involved in this vibration. And when a molecule absorbs a photon the photon's energy is added to this vibration and the atoms jiggle harder.
There is a second mechanism that applies to a few GH molecules, notably water. Molecules don't just vibrate internally, they also tumble and rotate. Some molecules are able to absorb energy from a photon and convert it into a change in the rotational motion of the entire molecule rather than internal vibration of the atoms.
Finally there is the movement of the molecule through space and the absorption of the energy and momentum from the photon contributes to changing that.
Because the energy the molecule absorbs is in the form of mechanical, energy of movement it is then easily able to be transferred to other molecules around it through collisions. Molecules in the air at sea level each undergo billions of collisions per second with other molecules, mainly th non-GH gases oxygen and nitrogen. So when a GH molecule has been energised by absorbing a photon, it will usually actually lose that energy through collisions. So the absorption ends up adding to the total pool of energy present amongst all the molecules in the air.
The main gases in the atmosphere, oxygen and nitrogen can't absorb or emit infrared photons so it might seem that the energy from the absorbed photon has been lost forever, never to be released. But thhis is not the case.
Because GH molecules can also be energised in collisions with other molecules. At any instant a certain percentage of the GH molecules in the air will be in a more energised state due to recent collisions with other molecules. Mostly they will be de-energised again by another collision - it is estimated that around 1 in 50 collisions between molecules cause changes in the internal vibrations of the atoms - but occasionally they will de-energise instead by emitting an IR photon.
So the molecule that absorbed the photon may not be the one that emits it later.
And the new, emitted photon goes of in a completely random direction, unrelated to the direction the original came from.....
... And after a short distance is likely absorbed by another GH molecule and the game starts all over again. It really is like a game of billiards in 3 dimensions and on a vast scale.
So, going back to your use of the fog analogy. It might have some merit if used carefully, strictly defined as an analogy for illustrative purposes only but it shouldn't be used to derive an understanding of the process.
And to be useful you need to introduce something missing from the simple fog analogy.
The fog is glowing!
Even when there is no headlight shining through it, the fog is glowing from its own internal energy.
If the idea of a gas glowing seems strange, look up at the sky. The Sun. A big ball of gas and plasma that is glowing very, very brightly. Well cooler gases glow as well, just not as brightly, and not in the visible spectrum, but in the infra-red.
Hope that helps some.
Thank you Glenn @313
I needed to understand what fog is, so I could understand why CO2 is not fog.
I guess that fog is made of tiny little balls of water, that have reflective surfaces.
I did not realize that Carbon Dioxide only absorbs and emits, but does not reflect.
This is a very helpful answer.
Glenn @ 308:
A good catch. I had realized that figure 7.3 in that source was not covering the peak absorption wavelengths - hence my reference to figure 7.2 where the right side shows much greater absorption.
Other references I had on hand gave absorption coefficients with units such as kg/m2. When using Beer's Law, you must be careful of units, and typically simple distance (e.g., metres) isn't one you'll see because distance isn't a measurement of the amount of gas - you need density, pressure, etc. to get the full picture. I wasn't about to try to figure it out late at night, and missed the context of figure 7.1. I had also looked at table 7.1 in that document, which gives several possible units for path length, including "cm" and "cm atm". I don't think either one of them is intended to be simple linear distance.
It is possible to measure gas amount in length units, but the length isn't ordinary distance. Take ozone for example, where a Dobson Unit (a common measure of total column ozone) represents a thickness of ozone of 10 µm, when compressed to standard tempeature and pressure. The WIkipedia page also calls that a "milli-atmo-centimeter", so I'm going to guess that the "cm atm" unit in table 7.1 is a similar measure. Thus, just because an absorption coefficient has units of length doesn't mean the path length is really distance or height.
The data from figure 7.1 does definitely appear to represent a physical path in the free atmosphere of 1 metre, though. They refer to 100 and 1000 mb as the pressure, and "typical CO2 concentration".
The PDF file I linked to appears to be just one lecture in a course on atmospheric radiation, and the course syllabus is available here. It looks like it has a lot of interesting material.
Jonathan:
What KR and Glenn said. Scattering and absorption/re-emission are different beasts.
Wikipedia actually has an article on Rayleigh Scattering, which in the atmosphere is the scattering caused by the air molecules themselves. Ditto for Mie Scattering, which in the atmosphere is caused by all the particulate matter. In fog, also add reflection (including internal - i.e., inside the water droplets). And in all cases, expect multiple scattering - light that is scattered many times, sometimes to the point where you can't tell up from down (white-out in a blizzard).
I put together A 20 Minute Video that attempts to explain some of my current thinking. I don't think I have any current questions...
KR, Thanks for the reference to Harries. But at $32 to read the article, or $199 to get a year's subscription to nature. I'm going to try to figure it out on the cheap.
[DB] Try here.
Jonathon
See my comments attached to your video.
I've found some errors in the original argument which I would like to address. I've not read all 318 posts, so it's possible that somebody has already corrected them.
1. The correct term is extinction, not saturation. Nothing is being saturated, the 15 micron IR band radiates from earth's surface and is absorbed to extinction by CO2 in the lowest level of the atmosphere (the elevation of extinction is around 500 feet).
2. The argument given here gives the false impression that the only IR that radiates into space comes from CO2 radiating at 15 microns from the top of the atmosphere. Let us refresh our memories on how earth loses heat:
a. blackbody radiation from the surface which radiates directly into space through the many windows in the spectrum where the atmosphere is transparent to IR.
b. blackbody radiation from cloud tops in the troposphere
c. quantum radiation from H2O molecules in the troposphere, at many different wavelengths
d. quantum radiation from CO2 in the stratosphere, at 15 microns
e. quantum radiation from O3 in the thermosphere, at 9.6 microns
The argument given here only addresses "d" and ignores "a-c" and "e". In fact, the vast majority of radiation to space from earth is from "a"
Another glaring error in the argument given here: it treats the radiation at the top of the atmosphere, I suppose from CO2, as if it were blackbody radiation. I say this because the argument specifically states that if the elevation of radiation is higher, the temperature becomes colder, so the amount of radiation is less. That would only be true for blackbody Planck radiatiors. CO2 is a gas and does not emit blackbody radiation. It emits quantum specific radiation, namely 15 microns, which according to Wiens law corresponds with around 200K. We all know, 200 K is very cold. If the elevation of this emitting layer of CO2 climbs higher, and therefore becomes colder, it makes the temperature closer to 200K and therefore the emission would be STRONGER, not weaker. The giant error in the argument is assuming that as the layer grows colder, it will emit less, when the opposite is true....as it gets closer to 200K the emission increases because 200K is the quantum specific temperature of maximum emission.
Satoh @329:
1) The term "saturated greenhouse effect" has a long history, and is well understood. "Extinction" refers to the point at which no radiation from the original source remains - which is a different concept. The greenhouse effect is claimed to be saturated by the misinformed due to the fact that radiation from the Earth's surface is extinguished in the 15 micron band at very low levels (which is a non-sequitur on a variety of grounds).
Confusing the two terms only confuses the issue, leading the gullible to believe that the greenhouse effect cannot become stronger with increased CO2 concentration (ie, it is saturated) because IR radiation from the Earth's surface at 15 micron is in fact extinguished low in the atmosphere. The two are not the same thing.
2) I do not believe the article leaves that impression. (Which, article by the way? I assume you mean the basic article.) However, to the extent that it does, it is only because it is discussing the claim that the greenhouse effect is saturated because IR radiation from the surface at 15 microns does not escape to space. Because that is the argument which is being responded to, of course attention is focussed on CO2.
Working through the list, you claim that "In fact, the vast majority of radiation to space from earth is from "a"", which is simply false. Here is the partition of energy flows by Kiehle and Trenberth, 2009:
As you will notice, only 40 W/m^2 out of 249 W/m^2 of the IR radiation to space comes directly from the planets surface. A further 30 W/m^2 comes from cloud tops. Of the remaining 179 W/m^2, all of it comes from the atmosphere, but most of it will come from water vapour. However, as the greenhouse effect works by replacing a high IR flux from the surface with a lower IR flux from the atmosphere at certain wavelengths, the lower the IR contribution from a given gas, all else being equal, the stronger the greenhouse effect caused by that gas. The argument that CO2 is of relatively little importance because it contributes relatively little to the total IR flux has the relationship exactly reversed, and shows a lack of understanding of the greenhouse effect.
3) (@320), first and most generally, you are in complete disagreement with line by line radiation models. They assume atmospheric gases are grey bodies and calculate emissions and absorptions spectral line by spectral line, and produce results, when compared to observations like this:
And like this:
If you will excuse me, I will accept such a well confirmed theory as that presented by LBL models over the bad theory of "random internet guy".
More specifically, you are using Wein's displacement law incorrectly. It points out the wavelength (or frequency) of the peak radiation for a given temperature of a black body. It does not indicate the temperature of peak emission for a given wavelength (or frequency). In fact, for black bodies at any given wavelength, the emission at that wavelength will be greater at higher temperatures in all cases:
(Wikipedia)
Because CO2 molecules in the atmosphere gain the energy that they emit by molecular collision, the intensity of radiation at the frequencies in which it radiates follow the black body laws. Therefore decreased temperature will decrease emission from CO2 at 15 microns even though it approaches the temperature at which 15 microns is the wavelength of peak emission for a black body. Indeed, it will continue to do so until molecular collissions occur at a frequency much less than the frequency of reemission of absorbed radiation, or until the temperature is much less than 200 K (so that thermal emission can be neglected).
Your first two graphs disagree with each other. The first graph says only 40 W/m2 out of 396 are emitted directly through atmospheric spectral windows, which is only 10%. The irradiance chart, on the other hand, clearly shows that much of the spectrum has high transmission of IR through the atmosphere, with the exception of a big CO2 band from 13 to 18 microns. Your radiance chart is incomplete by the way, because it only goes from 10 microns to 25, when the earth radiates from 7 to 100 microns. The complete chart spectrum, from 7 to 100, shows very little absorption by the atmosphere from 7 to 13 microns, and again very little absorption from 18 to 100, which is a big enough part of the spectrum for me to know that Trenberth's figure of 10% is extremely low. I don't believe it. The large amount of radiance to space from earth surface, through windows, is evidenced by the fact that IR images of earth taken from satellites show surface features such as continents and islands, lakes, etc, which would not be visible if 90% of the IR is absorbed by and re-emitted by the atmosphere. A 90% atmospheric interference of IR would mean the atmosphere is basically transleucent to IR, and IR cameras in satellites would not show continents, etc, but would show nothing but a featureless haze. I don't buy Trenberth's cartoon calculations at all.
Every time someone throws issues a challenge to Mr Curtis, I want to get pretzels, his science-powered takedowns are exquisite.
Satoh
"The irradiance chart, on the other hand, clearly shows that much of the spectrum has high transmission of IR through the atmosphere, with the exception of a big CO2 band from 13 to 18 microns"
This is a common misunderstanding. Take the region on the left of Tom's second graph, to the left of wavenumber 600. That is a region dominated by water vapour absorption/emission. And not that the intensity corresponds to a Planck curve with a temperature below 280 K. I.e, well above the surface.
What you are seeing there is not IR that has been transmitted through the atmosphere. Water vapour absorbs virtually all the IR leaving the surface. The transmittance is negligible. What is being measured there is IR radiated from higher in the atmosphere. It is not transmitted through it.
Siimilarly the CO2 region represents again near total absorption the reradiation at higher altitudes. Much higher than the altitude the signals from the water band are coming from so much colder. So the intensity is so much lower. Look at the spike in the center of the CO2 'notch'. That corresponds to an altitude so high that it is in the upper stratosphere where temperatures are actually warmer. The detailed shape of the CO2 'notch' is a direct reflection of the verticl temperature profile of the atmosphere.
Tom's 'graph' is actually taken from an observations based paper from 1970. It is one of three such graphs provided by that paper and covers a larger frequency range, but still ultimately limited by the operating range of the instrument on the Nimbus 3 satellite.
Your earlier comment "That would only be true for blackbody Planck radiatiors. CO2 is a gas and does not emit blackbody radiation. "is only partly correct. A gas does not radiate as a black (or even grey) body in that it does not radiate a continuous spectrum with a Planck Function shape. It only radiates over a narrower range of discrete frequencies. However, at those frequencies the intensity of the radiation emitted by the gas does match the strength of a black body at that frequency.
Yo are making a claim that the ability to discern continents in IR suggests relatively high transmission. However you haven't backed up that assertion.
Trenberth's 'cartoon calculations' aren't his. That paper amalgamates measurements from many different sources to produce that composit picture.
And Tom's pont about your misunderstanding Wien's Displacement Law is spot on. Look at his figure 2. Pick any wavelength and compare the magnitude of the different Planck curves shown. Where do any of them show a higher intensity for a lower temperature at any single wavelength? A cooler Planck curve always lies below a warmer one, at all wavelengths.
Satoh
"The argument given here only addresses "d" and ignores "a-c" and "e". In fact, the vast majority of radiation to space from earth is from "a"
Not true.
"a. blackbody radiation from the surface which radiates directly into space through the many windows in the spectrum where the atmosphere is transparent to IR."
There aren't many windows. There is a recognised region around 10-11.5 microns labelled the Atmospheric Window where tranmission through the atmosphere is nearly total. Across the rest of the spectrum absorption is nearly total. What we are seeing in graphs like what Tom has shown is predominantly not transmission. It is reradiation.