OA not OK part 20: SUMMARY 2/2
Posted on 25 August 2011 by Doug Mackie
This is part 2 of 2 summarising out series about ocean acidification. The posts: Introduction, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
During July and August we posted an 18 part series to introduce the basics of ocean acidification chemistry. In this post we summarise posts 11-18. We have distilled each post to the bare minimum (100 words average). Naturally a lot has been lost; please refer to the original posts.
Part 11: Did we do it? Yes we did!
The International Energy Annual reports that globally, 603 billion tons of CO2 were released from 'consumption and flaring of fossil fuels' between 1980 and 2006 giving an expected change of 77 ppm CO2 in the atmosphere. BUT atmospheric CO2 increased by only 43 ppm. 270 billion tons of CO2 is missing.

Part 12: Christmas present
4 parameters describe the marine CO2 system. If you know any 2 you can calculate the other 2.
Total dissolved inorganic carbon (CO2, H2CO3, HCO3–, and CO32–), Total Alkalinity, pH, and CO2 partial pressure. The partial pressure of CO2 in the atmosphere changes seasonally as plants photosynthesize. Since pH is influenced by the partial pressure of CO2, the pH of seawater also shows seasonal fluctuations. Though the rate of change is not even around the world, all the data agree: ocean pH is decreasing.
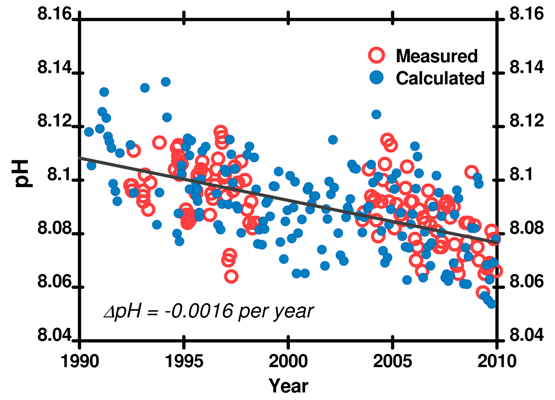 Figure 6. pH recorded at the Hawaii Ocean Time Series (HOTS) station. Data here.
Figure 6. pH recorded at the Hawaii Ocean Time Series (HOTS) station. Data here.
Part 13: Polymorphs: The son of Poseidon
Biological calcium carbonate comes in two main forms, or polymorphs: aragonite and calcite.
Aragonite is found in pteropods (small free swimming snails) and corals. Calcite is found coccolithophores and foraminifera. Molluscs can use either or both polymorphs.
![]()
Part 14: Going down
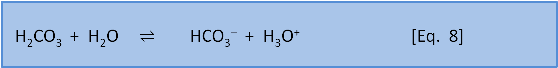
Photosynthesis in the ocean is confined to the upper 200 m or so where sunlight reaches. Below that depth falling organic matter gets eaten by bacteria (producing CO2). Making CO2 produces H3O+ via Eq. 7 & 8. Dissolution of calcium carbonate via Eq. 4 increases the pH slightly (by removing this acid).

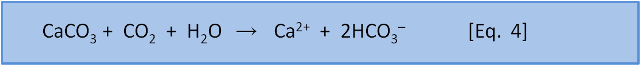
As depth increases, the pH does not recover to the surface value because there is more respiration (producing acid) than shell dissolution (consuming acid). Thus, total dissolved carbon increases with depth (due to respiration) but carbonate decreases with depth because it is a function of pH which decreases with depth.
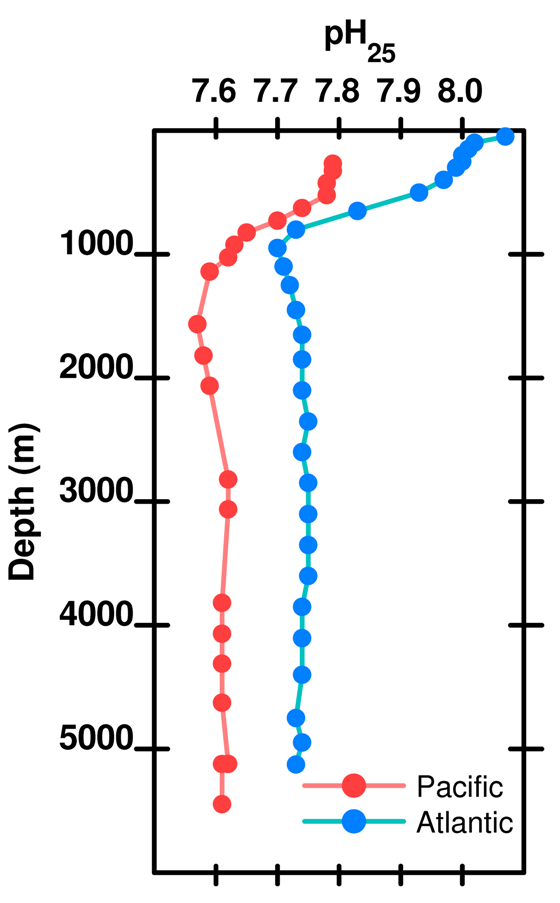 Figure 13. pH (at 25 deg C) as a function of depth in the Atlantic and Pacific oceans. Data .
Figure 13. pH (at 25 deg C) as a function of depth in the Atlantic and Pacific oceans. Data .
Part 15: No accounting for taste
The common ion effect describes the way the dissolution of a salt is inhibited if the solvent already contains an ion in common with the salt (e.g. the solubility of CaCO3 decreases in water containing lots of Ca2+ ions). Similarly, dissolution is enhanced if the concentration of one of the ions, say carbonate, is low (e.g. the dissolution of CaCO3 increases in water if CO32- has been removed by an external factor).
Part 16: Omega

If Ω < 1 the solution is undersaturated and dissolution occurs. If Ω > 1 the solution is supersaturated and dissolution does not occur. For a line at Ω = 1, the depth at which this line crosses the actual Ω gives the depth below which aragonite and calcite dissolve. This is the saturation depth. A poetic image used is to compare Ω to a mountain snow line.
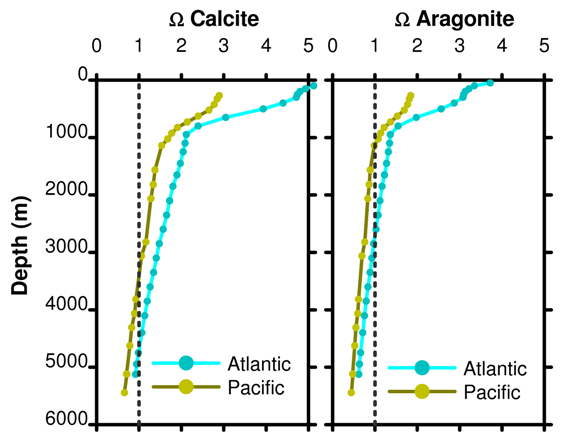
Figure 15. Depth profile of Ω for calcite and aragonite as a function of ocean basin.
Adding CO2 to the surface ocean will cause overall carbon to increase, but also causes the relative fraction of carbonate to decrease. In turn this decreases Ω so the profiles in Figure 15 to move to the left and the saturation horizon moves towards the surface.

Part 17: Pumping currents
Two processes move CO2 from the air-sea boundary into deep waters:
1. Biological pump: Photosynthesis transforms the CO2 into big organic particles that sink rapidly. As the particles fall to the bottom, they get eaten by bacteria, producing CO2 in deep waters.
2. Ocean circulation: The high volume but slow (100s-1000s of years) circulation called the thermohaline ocean circulation swaps surface water with deep water. Added CO2 remains in surface waters until the slow ocean circulation turns the surface waters over. This means the surface ocean is undergoing acidification to a much greater extent than the deep ocean.
Part 18 : Been this way before
We know CO2 levels have been high in the past. What makes this time different?
1. We are outside normal CO2 levels
During an ice age, CO2 is about 180 ppm; during an interglacial CO2 is about 280 ppm. Atmospheric CO2 is currently 394 ppm - 100 ppm above the normal recent maximum.
2. Changes are happening faster than normal.
At the end of an ice age, the 10oC temperature change and the 100 ppm CO2 change occur over 10,000-15,000 years. CO2 is currently increasing at >2 ppm per year – over 100 times faster than the glacial-interglacial cycle.
The ocean carbonate buffer system will not restore the ocean to a preindustrial state. Instead the ocean, and the climate of the Earth as a whole, will converge towards a new stable state. We still don't know precisely what that state will be.
That's all folks. An index with a 2 line description of each post should now greet you when you click the "OA not OK" button at the top left. The booklet will be available in a few days and will be announced in a very brief post.
Written by Doug Mackie, Christina McGraw, and Keith Hunter. This post is the final summary of a series about ocean acidification. Other posts: Introduction, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.































 Arguments
Arguments
































I think I've just bumped into one of the concepts this series "skips lightly over", and I'm hoping you will kindly help me to crawl around it. The concept is buffer theory, and the reason I only think it's my obstacle is that I encountered it in this "simple description" of the ocean's importance for climate, written by an oceanographer:
"...it is also clear that the ocean’s capacity for carbon dioxide is limited, as indicated by its increasing acidity. This is most extraordinary since the ocean has always been considered to be strongly buffered against acids and alkalis alike;"
To me, a buffer is a space that separates two things for safety, as in "buffer zone" or a buffer in a schedule. After a bit of googling around I'm guessing in chemistry it's a process rather than a space and that buffer theory spells out how buffering mechanisms (mechanisms?) work. I also suspect it's such a fundamental concept that even a simple explanation requires a boatload of technical terminology, but I hope I'm wrong about that.
FYI the simple description is being written for a regular "climate column" we're doing for our local newspaper. Our readers' assumed level of knowledge is about year 8 school science 40 years ago. It's a great opportunity, but it would be very easy to scare the editor, never mind the readers.
I'm not asking you to write to the level we're aiming for, but I'd be very grateful for an explanation that makes sense to me when I'm translating for my oceanographer. Can you help?
Thanks for the series and for this site.
Susanne - I'll reply without using equations, but you really need to follow the equations to understand this in greater depth:
1. The notion of akali and acid, as it pertains to ocean acidification, is irrelevant. Ocean acidification is the process of increasing the hydronium ion concentration in seawater (lowering pH). It has nothing to do with the concept of neutral pH. The oceans will very likely remain above a pH of 7 (neutral), but can still be highly corrosive to marine life that make their shells/skeletons (calcifiers) from calcium carbonate (chalk). If someone drags up the issue of neutrality, or acids and akali, you can be certain they don't understand the fundamentals and are probably playing the troll.
2. The oceans become corrosive to marine calcifiers because increasing the atmospheric CO2 partial pressure forces more CO2 to dissove into the oceans and leads to a series of reactions which diminish the concentration (activity actually) of carbonate ions in seawater. These carbonate ions are one of the key building blocks of the calcium carbonate shell/skeleton. So, although pH (the concentration of hydronium ions) is critical for a number of biological chemical reactions, it's not lower pH per se that makes the oceans corrosive to marine calcifiers - it's the decline in carbonate ion activity.
3. Now on to buffering. The carbon chemistry of the oceans (the series of reactions between the various forms of carbon dissolved in seawater) means that the oceans buffer (act against) the lowering of pH. So yes the oceans are buffered to some extent. The equation in which a hydronium ion combines with carbonate to form bicarbonate is the one which buffers against ocean pH being even lower than it is, because the "extra" hydronium is no longer free in solution - it is now bound with the carbonate ion to form bicarbonate. If this reaction did not take place the hydronium concentration of seawater would be higher and, therefore pH lower. But this is the very same reaction which lowers the carbonate ion concentration in the oceans and makes them corrosive to marine calcifiers!
4. Two other buffering mechanisms exist, but they operate on such long timescales that they are of no use to humanity, or the marine life put at threat by ocean acidification. One is the supply of alkalinity back to the oceans through the chemical weathering of silicate rocks through rainfall. And the other is the dissolution of carbonate sediments on the ocean floor when the oceans become corrosive. These both operate on 10-100,000-year timescales, so won't be of any use to us.
5. This slow buffering is why the oceans became corrosive in Earth's past when CO2 increased in a geologically abrupt manner, but did not when the CO2 content of the atmosphere increased slowly, or was sustained at high levels for long geologic intervals - the system is able to readjust and move itself back toward an ocean condition which is suitable for marine life through these buffering processes. Perhaps the best example of this are the White Cliffs of Dover. These are huge chalk deposits formed by coccoliths (microscopic marine life that build their shells from calcium carbonate) during the Cretaceous - a time of very high atmospheric CO2 and much warmer global temperature. Because there was no geologically-abrupt spike in CO2 the oceans did not become corrosive, despite the low pH.
6. Perhaps the best way to skewer bogus claims about ocean acidification is show what is actually going on today - marine life is beginning to be eaten away by corrosive seawater. The pic below is of a pteropod (sea butterfly) captured from waters around Antarctica recently:
The pic is from this peer-reviewed paper: Extensive dissolution of live pteropods in the Southern Ocean - Bednarsek (2012).
Rob, Thank you very much. I didn't expect such a quick or detailed response, especially as I'm sure that explaining without equations is uncomfortable.
Thanks again.
You're welcome Susanne. I'm actually very comfortable explaining things without equations, I just thought if you remembered a couple of the basics it would stick in your memory, so that you would have the confidence to explain it to others.
I have quite a few posts on ocean acidification coming up over the next few months too, by the way.
The pic below is of a pteropod (sea butterfly) captured from waters around Antarctica recently:
I dont see a ph reading do you happen to know how alkaline the water was where this pterapod was collected??
Read the paper linked to in the comment.
@27 yeah easier said than done when you have a bank account.
After the ice age I guess the oceans outgassed co2 as the seas would have been almost saturated due to temperature? wouldnt the sea then be closer than we are today to ph neutral?
Vonnegut - "@27 yeah easier said than done when you have a bank account."
......Or know how to use Google apparently. A free copy is available here: Extensive dissolution of live pteropods in the Southern Ocean - Bednarsek (2012).
As for CO2 in the Southern Ocean during the last ice age - that's somewhat of a mystery. How was it stored? What caused it to be released back into the atmosphere? We don't yet have suitable answers for that.
If, as suggested, this CO2 was stored in the Southern Ocean (in whatever form) and then vented into the atmosphere as the Earth warmed it would have become well-mixed in the air and thus raised the partial pressure of CO2 in the atmosphere. Consequently more CO2 would dissolve into the oceans - lowering pH in the surface ocean.
It only seems counterintuitive to you because you are (I suspect) thinking the CO2 (supposedly) stored in the Southern Ocean during the last ice age is well-mixed throughout the surface ocean. That's not correct, and is not the idea put forward.
The reason Im questioning is because ph and temp are very variable in the oceans, creatures are well adapted to that variability, for instance ph can and does change with the tides sometimes over 0.8 ph, there is also the fact more co2 is more food for algae which coral need to survive. The drop in ph is going inside the working range not outside it.
vonnegut, in your post at 31 you make several unsupported assertions, please provide references to support the contention that:
(1) creatures are well adaptend to variability in pH. I don't doubt that some creatures are well adapted, but that does not mean that all are well adapted. If you want to argue that this is not a problem, it is incumbent on you to provide the evidence that changes in pH will not result in ecologial change.
(2) More Co2 means more food for algae which coral need to survive. You need to show that CO2 is the rate limiting factor for this to be relevant.
(3) drop in pH is going inside the working range not outside it. This is a very specific claim, please provide references detailing the "working range".
vonnegut
(1) that is one location only, it is also variation over one day, that sort of variability does not mean that a change of that size in mean pH is tolearble. In the U.K. temparatues often vary by 15 degrees C or more during the course of 24 hours. That does not mean that all of our fauna and flora are tolerant to a change in average temperatures of 15 degrees C.
(2) if you increase the amount of oxygen in the air to 100% do we use all of it? No. Algae do have requirements for other nutrients, they are not wholly composed of carbon, oxygen and hydrogen.
(3) again that applies for one location only, and as I pointed out the variability is diurnal, so it is not a reasonable guide to the "working range".
You have not yet provided evidence that actually justifies your position.
BTW, the first line of the abstract of the paper voneggut mentions reads as follows: "Ocean acidification is projected to shift coral reefs from a state of net accretion to one of net dissolution this century", is this projection explicitly refuted anywhere in the paper? Not as far as I can see.
(1) yes its one location among the barrier reef one of the most diverse sources of tropical aquatic life on the planet , If Uk flora and fauna are not tolerant to 15c change they wouldnt be in the UK so I would call them tolerant.
(2) No the planet would explode but thats not relevant. co2 is food for algae if its not used by them directly, its used in building the reefs
(3) Its the sea, its alkaline, across the globe it varies between those figures.
www.ukmarinesac.org.uk/activities/water-quality/wq9_6.htm from around the UK look at the differences
@35
It uses the scientific get out clause "may"
I wonder how they can tell the difference between natural and anthropogenic co2?
vonnegut
(1) I said much of UK flora and fauna would be unable to withstand a 15 degree change in MEAN temperature, of course they can and do withstand that sort of variability over the course of a day. I suspect the last time mean U.K. temperatures were 15 degrees lower than today, much of the country was under an ice sheet. Do you think that U.K. flora and fauna could withstand that?
(2) you have just completely ignored the point on this one. I said that algaes need more than carbon, oxygen and hydrogen to survive, you have not addressed that point in any way.
(3) Ph may vary around the globe, but then again so does the flora and fauna, which are adapted to local conditions, so you still haven't established that OA will not take sea creatures out of their "working range". Lets make it easy and stick with the barrier reef, what is the working range for the barrier reef in terms of annual mean pH?
vonnegut @ 37 Sorry that is plain evasion, I asked a straightforward question, to which the answer is "no, the opening sentence of the abstract is not refuted by anything in the paper", but you were not able to acknowledge that. This gives the strong impression that you are just trolling. Life is too short to waste discussing science with people who can't admit to shortcomings in their arguments. I suggest we all ignore vonnegut.
(1) Sorry I missed the "mean" but a 15 degree change in mean temp is not the same as a change in ph changes we are discussing. thats equivalent of the sea becoming acidic which its not.
(2) Ive ignored the point because its not relevant, whatever else they need is there already obviously or we wouldnt get algal blooms would we?
(3) extremes of ph will have more effect on life than a change in mean
nsf.gov/news/news_summ.jsp?org=NSF&cntn_id=130129&preview=false
iopscience.iop.org/1748-9326/7/2/024026/article
Some fauna seems to do well with more food available
@ 39 No it isnt, does it need to be? "may" sounds about as vague as it gets. No evidence it will or it wont.
vonnegut
(1) again you have missed the point that saying that a coral reef can withstand a particular range of pH over the course of a day does not mean that they can withstand the more permanent change of that size that would result from OA. You still have not addressed this point.
BTW "Acidification" means to become more acidic, if you go from a pH of 12 to a pH of 11, that is more acidic, and less alkaline, even though both pHs are alkaline. Please no quibbling on this point, it has been addressed repeatedly.
(2) You wrote "(2) well apart from sunlight and water what else do algae need?" they need nutrients other than carbon, oxygen and hydrogen, just like any other organism consisting of proteins. Thus the factor limiting growth of algae is not necessarily carbon dioxide. My objection is relevant as it is the answer to your question.
(3) you STILL have not specified the annual mean pH gvining the working range for coral and your answer is yet more evasion.
@41 "no it isn't does it need to be". Yes, that is the way scientific discussion works, if a direct question is posed, you give a direct answer, and admit when you are in the wrong rather than engaging in evasion. If you want to have a rhetorical argument instead, then evasion is sufficient, but you will find that it won't go down well here.
Scientists don't use "may" to be deliberately vague, they use "may" when the evidence is anything but completely unequivocal. The article you post states that OA is projected to cause coral dissociation, and contains no statement that casts doubt on that projection. This means that trying to use it to suggest otherwise is quote mining.
To give another example of vonnegut's quote mining, (s)he uses the web page here to support the claim "extremes of ph will have more effect on life than a change in mean". However the sub-title of the article is "Corals living in more acidic waters are healthy, but is the situation one-of-a-kind?" and later in the article it says:
.In other words, the reef at Palau is resistant to OA because of its geology and if you took the corals to some other location where the acidity was due to other factors they may not survive.
Thus the article provides no real support for the contention made, whatsoever. Voneggut should be ashamed of him/herself for stooping to that sort of behaviour.
(1) You misunderstood my reference, I know they describe the ph drop as becoming more acidic. It was reference the huge disparity between 15c mean temp change being the same as water going from alkaline past neutral to acid.
Its not about a particular reef and Im not suggesting all creatures can cope with changes such as those living around the barrier reef can, The ocean is an immense place with much variability in so many aspects. A whiting fish for instance would experience a change in ph from 8 to 6 and saltwater to esturine youre telling me that a change in mean ph of 0.1 will matter to him?
(2)we are discussing the effects of more co2 not a lack of nutrients for algae , Im guessing algal blooms happen because there is an excess of something. Im guessing its co2, it could be something else.
(3)You may know where to find the Annual mean ph you may not, does this mean you will find them or you wont?
vonnegut
(1) You claimedthat the diurnal range of pH from 8.6 to 7.6 at Lady Elliot Island reef flat (Great Barrier Reef, Australia) answered by challenge " If you want to argue that this is not a problem, it is incumbent on you to provide the evidence that changes in pH will not result in ecologial change.". That coral reef us unquestionably adapted to that range of DIURNAL pH, just as UK fauna and flora is well adapted to a 15 degree change in DIURNAL temperature. So the 15 degrees is not a big change in diurnal temperatures, for the U.K it isn't at all unusual. It would however be a big change in MEAN temperatures. You STILL have not established that OA that would result in large long-term changes in MEAN ocean pH would be tolerable for ocean flora and fauna that are adapted to the mean pH in their current environment, so the Lady Elliot island reef figures do not answer the challenge.
(2) You still have not addressed the point that CO2 may not be the rate limiting factor for growth of algae in coral reefs. If you want to find out what causes algal blooms, you could try looking it up on the WWW using google (e.g. Wikipedia - hint nitrogen is also needed to make proteins as well as carbon, hydrogen and oxygen and notrogen and phosphorus to make DNA).
(3) It is your responsibility to be able to provide support for your position, not mine.
voneggut wrote "It isnt 'resistant' to OA it creates its own and its still connected to the ocean so whatever the mean ph is , its going to be on the receiving end."
this is pathetic quibbling, if the Palau reef creates its own OA it must also be resistant to OA as otherwise it would be poisoning itself. You are just trying to evade the fact that the reference you provided (again) did not actually support your argument. If you had more sense you would have just let it drop, rather than draw attention to that fact once more.
(1) I think most things in the ocean will be just fine, some even better for the extra co2 for shell making and food production, you dont .
(2) Youre fixated on algae im sure they wont pass up a free meal if one passes.
(3) you didnt read 3 did you? theres a clue in it ;)
@47 you are saying OA will destroy reefs Palau is a prime example of how it wont.
I think youre confusing extra co2 with pollution we have plenty of pollution problems more damaging to reefs than co2.
vonnegut@48
so we are back to where we started with unsupported assertions.
(1) Had you read part 1 of the summary on OA is not OK, you would know that although there is additional carbon in the oceans, it is not in the form that can be used for shell making (so I do have reason to think that OA is not OK, your opinion that most things in the ocean will be just fine on the other hand is unsupported opinion):
(2) Try living for a year on as much refined sugar and water as you can eat and drink (which will supply all of the carbon, oxygen and hydrogen you can use) and see how you feel afterwards. If you don't have suitable supplies of nitrogen and phosphorus you will not grow or flourish. The algae will only make use of the "free meal if one passes" if carbon is the rate limiting factor.
"Youre fixated on algae", this is a well known rehtorical technique of trying to wind your opponent up and irritate him. Sorry won't work on me, been discussing climate online long enough that this sort of nonense doesn't bother me. It does however demonstrate that your main interest is rehtorical rather than scientific. It was you that brought up the subject of algae, I am just asking you to justify your assertions on that subject.
(3) I did read it, and as I demonstrated it didn't support uour contention. Blustering about it doesn't change that. Again your tone seems intended to irritate, again it failed. If you have evidence, give a specific quote from the paper (and to show that you are not quote mining, mention any quotes from the paper that would not support your contention).
@47 no, I am saying that it doesn't support your assertion because it is a discussion of one reef that is deeply unrepresentative of the worlds reefs in general.
"I think youre confusing extra co2 with pollution we have plenty of pollution problems more damaging to reefs than co2."
This is a transparent attempt to divert the discussion away from OA. No, I am not talking about pollution, I am talking about OA.
(1) so whats going to happen to those creatures in Palau being that theyre creating a more acidic environment? Surely the extra co2 pressure is imparted on their piece of ocean too? They must run out of carbonates soon?
(2)you can grow algae in abundance in a bucket of clean water and sunlight, and get it to flourish with co2 injected without knowing what else it needs.
(3)It wasnt intended to do anything except highlight the way 'may' can be used to demonstrate doubt and could be substituted with 'may not'.and still demonstrate doubt.
Saying Palau is deeply unrepresentative is a surprise, testing creatures in a lab is unrepresentative sometimes. I dont know for sure but I guess that the species in there are the same as other species locally that dont live in that particular reef, being that the creatures themselves are making the water more acid means its happening very fast? apart from the fact that its a great place to study the effects of OA even if they are created by the creatures within.