OA not OK part 20: SUMMARY 2/2
Posted on 25 August 2011 by Doug Mackie
This is part 2 of 2 summarising out series about ocean acidification. The posts: Introduction, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
During July and August we posted an 18 part series to introduce the basics of ocean acidification chemistry. In this post we summarise posts 11-18. We have distilled each post to the bare minimum (100 words average). Naturally a lot has been lost; please refer to the original posts.
Part 11: Did we do it? Yes we did!
The International Energy Annual reports that globally, 603 billion tons of CO2 were released from 'consumption and flaring of fossil fuels' between 1980 and 2006 giving an expected change of 77 ppm CO2 in the atmosphere. BUT atmospheric CO2 increased by only 43 ppm. 270 billion tons of CO2 is missing.

Part 12: Christmas present
4 parameters describe the marine CO2 system. If you know any 2 you can calculate the other 2.
Total dissolved inorganic carbon (CO2, H2CO3, HCO3–, and CO32–), Total Alkalinity, pH, and CO2 partial pressure. The partial pressure of CO2 in the atmosphere changes seasonally as plants photosynthesize. Since pH is influenced by the partial pressure of CO2, the pH of seawater also shows seasonal fluctuations. Though the rate of change is not even around the world, all the data agree: ocean pH is decreasing.
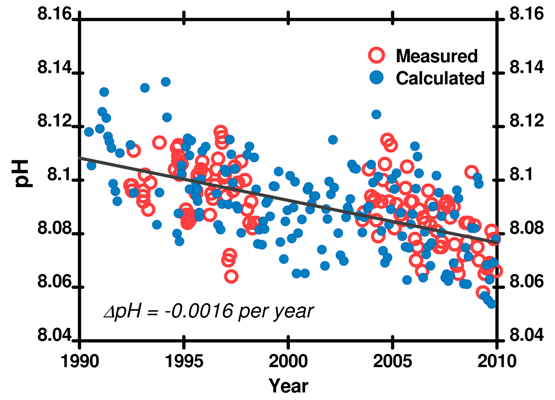 Figure 6. pH recorded at the Hawaii Ocean Time Series (HOTS) station. Data here.
Figure 6. pH recorded at the Hawaii Ocean Time Series (HOTS) station. Data here.
Part 13: Polymorphs: The son of Poseidon
Biological calcium carbonate comes in two main forms, or polymorphs: aragonite and calcite.
Aragonite is found in pteropods (small free swimming snails) and corals. Calcite is found coccolithophores and foraminifera. Molluscs can use either or both polymorphs.
![]()
Part 14: Going down
Photosynthesis in the ocean is confined to the upper 200 m or so where sunlight reaches. Below that depth falling organic matter gets eaten by bacteria (producing CO2). Making CO2 produces H3O+ via Eq. 7 & 8. Dissolution of calcium carbonate via Eq. 4 increases the pH slightly (by removing this acid).

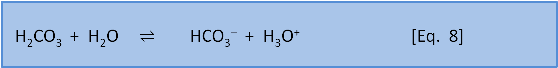
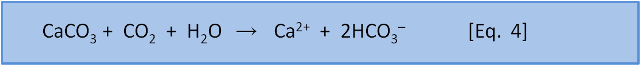
As depth increases, the pH does not recover to the surface value because there is more respiration (producing acid) than shell dissolution (consuming acid). Thus, total dissolved carbon increases with depth (due to respiration) but carbonate decreases with depth because it is a function of pH which decreases with depth.
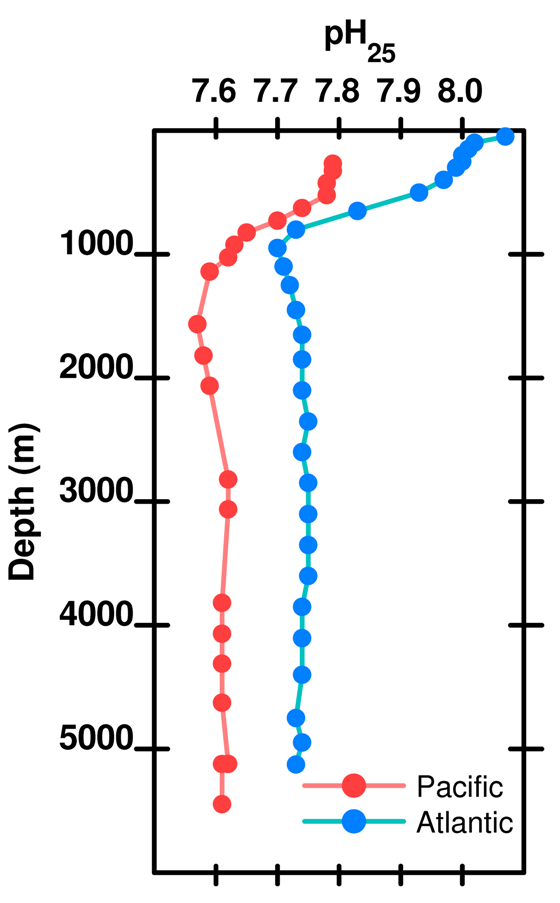 Figure 13. pH (at 25 deg C) as a function of depth in the Atlantic and Pacific oceans. Data .
Figure 13. pH (at 25 deg C) as a function of depth in the Atlantic and Pacific oceans. Data .
Part 15: No accounting for taste
The common ion effect describes the way the dissolution of a salt is inhibited if the solvent already contains an ion in common with the salt (e.g. the solubility of CaCO3 decreases in water containing lots of Ca2+ ions). Similarly, dissolution is enhanced if the concentration of one of the ions, say carbonate, is low (e.g. the dissolution of CaCO3 increases in water if CO32- has been removed by an external factor).
Part 16: Omega

If Ω < 1 the solution is undersaturated and dissolution occurs. If Ω > 1 the solution is supersaturated and dissolution does not occur. For a line at Ω = 1, the depth at which this line crosses the actual Ω gives the depth below which aragonite and calcite dissolve. This is the saturation depth. A poetic image used is to compare Ω to a mountain snow line.
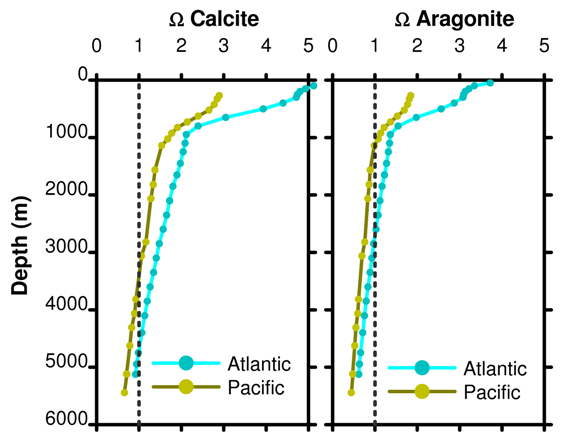
Figure 15. Depth profile of Ω for calcite and aragonite as a function of ocean basin.
Adding CO2 to the surface ocean will cause overall carbon to increase, but also causes the relative fraction of carbonate to decrease. In turn this decreases Ω so the profiles in Figure 15 to move to the left and the saturation horizon moves towards the surface.

Part 17: Pumping currents
Two processes move CO2 from the air-sea boundary into deep waters:
1. Biological pump: Photosynthesis transforms the CO2 into big organic particles that sink rapidly. As the particles fall to the bottom, they get eaten by bacteria, producing CO2 in deep waters.
2. Ocean circulation: The high volume but slow (100s-1000s of years) circulation called the thermohaline ocean circulation swaps surface water with deep water. Added CO2 remains in surface waters until the slow ocean circulation turns the surface waters over. This means the surface ocean is undergoing acidification to a much greater extent than the deep ocean.
Part 18 : Been this way before
We know CO2 levels have been high in the past. What makes this time different?
1. We are outside normal CO2 levels
During an ice age, CO2 is about 180 ppm; during an interglacial CO2 is about 280 ppm. Atmospheric CO2 is currently 394 ppm - 100 ppm above the normal recent maximum.
2. Changes are happening faster than normal.
At the end of an ice age, the 10oC temperature change and the 100 ppm CO2 change occur over 10,000-15,000 years. CO2 is currently increasing at >2 ppm per year – over 100 times faster than the glacial-interglacial cycle.
The ocean carbonate buffer system will not restore the ocean to a preindustrial state. Instead the ocean, and the climate of the Earth as a whole, will converge towards a new stable state. We still don't know precisely what that state will be.
That's all folks. An index with a 2 line description of each post should now greet you when you click the "OA not OK" button at the top left. The booklet will be available in a few days and will be announced in a very brief post.
Written by Doug Mackie, Christina McGraw, and Keith Hunter. This post is the final summary of a series about ocean acidification. Other posts: Introduction, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.































 Arguments
Arguments
































vonnegut, You will note that I pointed out that you had made a statement that was factually incorrect, namely " I think most things in the ocean will be just fine, some even better for the extra co2 for shell making and food production," (emphasis mine), which is directly contradicted by the part 1 of the summary.
There is no point in discussing science with someone that can't admit when they are wrong, so I shall leave it at that unless:
(i) you can show that Doug Mackie's chemistry is incorrect and that OA will make more CO2 available for shell making (and that of the many other researchers who have published papers on OA)
or
(ii) you admit that you were incorrect and that the additional CO2 won't be useful for shell making.
There is nothing wrong with making incorrect statements, but there is everything wrong with not being able to admit it.
Co2 for shell making...indirectly not directly. I saw the arguments posted on the first few pages, dont need to revisit thse do we?
Is the missing co2 reacting with all the limestone across the globe?
Ive not seen any science where marine animals have been grown in a carbonate saturated and co2 heavy atmosphere to know if its true or not. Sorry to say it bu Palau comes close to an experiment doesnt it?.
vonnegut wrote "Co2 for shell making...indirectly not directly. I saw the arguments posted on the first few pages, dont need to revisit thse do we?"
No, I didn't think you would admit you were wrong, I didn't think you would be able to prove Doug Mackie wrong either, and oddly enough you didn't. The fact that you blustered on anyway demonstrates beyond reasonable doubt that you are not here for a rational discussion of the science and are just trolling.
I didnt know I was disagreeing with anyone. I wasnt aware the sea was in a state of saturation regarding carbonates.
Anyway I was wrong, please stop accusing me of trolling Im trying to learn thats all.
vonnegut, I am happy to hear that trolling was not your intention, however your behaviour on the thread so far is just what you would expect to see from someone who was trolling, so perhaps you may want to revise your posting style. Science is a search for the truth, so nobody here is going to give you a hard time if you ask specific direct questions to help you understand the arguments, and give direct answers to direct questions.
Is the missing co2 reacting with all the limestone across the globe?
Ive not seen any science where marine animals have been grown in a carbonate saturated and co2 heavy atmosphere to know if its true or not.do you?
Sorry to say it bu Palau comes close to an experiment doesnt it?
vonnegut, it might be best to deal with one question at a time. What "missing CO2" are you referring to and what sort of reaction with limestone do you have in mind? I suspect this might not be the most appropriate thread for this particular question.
Its in part 6 of the part 1 summary, It isnt clear how much co2 is being consumed by natural rocks.
[PW] Vonnegut, this will be your first and *last* warning, from me: your repeated violations of the Comment Policy of this blog will no longer be tolerated. do it again, and your subsequent posts will be deleted in their entirety, and no explanation will be given. You've been repeatedly warned, yet persist in sloganeering. Cease.
The quoted paragraph seems pretty clear that the amounts involved are rather small. There is about 400ppmv CO2 in the atmosphere at the moment, so taking it all out in 3,500 year is about 0.1ppmv per year on average. The rate at which atmospheric CO2 is currently rising is about 1.5-2 ppmv IIRC, and that is about half of what anthropogenic emissions are actually contributing to the atmosphere. So without considering the effects of calcification a rough estimate might be that it accounts for 5% of anthropogenic emissions.
If we were to stop all emissions today, atmospheric CO2 levels would fall rapidly for 60 years or so until the atmosphere had equilibriated with the oceans, then more slowly as the upper layers of the ocean equilibriate with the deep ocean and but the full return to "pre-industrial" equilibrium will take tens to thousands of year to achive, largely by chemical weathering. See the work of David Archer (I've probably explained that badly and maybe have some details inaccurate, but the paper is a good one).
Vonnegut, are you really arguing that the fact some sea creatures can survive daily swings from 8.6 to 7.6 pH means that those same creatures will be able to survive daily swings from 6.6 to 7.6 pH after ocean acidification?
@61 in another 400 years? its dropped 0.1 in 200
Regardless of how long it takes. You wrote, "Lady Elliot Island reef flat (Great Barrier Reef, Australia) can range from preindustrial values pH 8.6 to future ocean acidification scenarios pH 7.6) over the course of a day."
This seems to argue that the ability to survive shifts of 1 pH over the course of a day indicates the ability to survive a 1 pH shift in average daily acidity. It should be obvious that there is no logical connection between the two.
Is there a citation for the corals being a source of co2? Ive seen the question asked but not seen a reply.
Ok Ive found a few papers confirming it.
The interactive on this site is missing something I guess?
www.whoi.edu/oceanus/feature/a-quest-for-resilient-reefs
[JH] Which "interactive" are you referring to? Exacrtly what is it missing ?
The multimedia interactive on the right, it shows the h ions leaving the shell but no co2
The interactive is correct as far as it goes - it is only concerned with the chemical processes for shell formation. It doesnt go on to show what happens next to seawater chemistry as result. When I first looked at this, I thought shellfish farming gave a way to doing CO2 sequestration, but sadly no.
2 HCO3- < --> CO3-- + CO2 + H2O
gets pushed to the right.
vonnegut wrote "Especially as co2 is such a bad guy."
Please keep the discussion factual and avoid this sort of rhetorical quip. All you will achieve is irritating people who would otherwise be only too keen to have a civil discussion of the science with you. Please give it a rest, we've all seen it before, and it does you no credit whatsoever.
Vonnegut, I'd say you're close to being permanently bore-holed. Wading through your na-na-boo-boo rhetoric to try to address whatever point you're trying to make or understand whatever question you're trying to ask is difficult, even more so since when the point or question arrives, it's more than likely irrelevant or based on a simple misunderstanding of the physics/chemistry involved.
Your understanding of the science is made plain in your last comment: "Especially as co2 is supposed to be adding to the warming of the planet by its excess."
Perhaps you'd better start at a more basic level and demonstrate why you doubt that CO2 absorbs/emits radiation at various pressure-broadened bands in the thermal infrared range, the range within which the sun-warmed Earth emits.
You're attempting to kill a tiger by pulling out (or attempting to) the hairs in its tail, one by one, and doing it with blinkers on.
All: The three most recent comments by Vonnegut were nothing more than argumentative sloganeering and were therefore deleted.
All: Vonnegut's most recent comment was off-topic and was therefore deleted. It also bordered on being a moderation complaint which is also banned by the SkS Comments Policy.
JH Feel free to prune back any of my posts you deem to be now unnecessary.
I'd be interested to know why it is possible for species of freshwater mussels and clams to calcify and produce shells at pH 4-5, whereas it will be difficult for saltwater species at pH 7.5?
[Rob P] - see this recent SkS post on ocean acidification - Corrosive Seawater, Not Low pH, Implicated As Cause of Oyster Deaths. Producing shells is somewhat problematic when the marine organism is dead as a result of ocean acidification (carbonate undersaturation).
Factor in the knowledge that ocean acidification is implicated as a kill mechanism in three of the 5 major extinction events, and that current ocean acidification is proceeding at a rate that is likely unprecedented in 300 million years, and there are legitimate reasons to be concerned.
This paper discusses the process for fresh water mussels. In short, they have evolved for it and the organisms pay an energy cost to do so. Like much about AGW, it is not the absolute temperature or the acidity that is the issue - it is the rate of change.
If the intent of your question is suggest that OA cant be that bad, then a quick search of google scholar on effects of ocean acidification will yield numerous papers documenting observed issues. I'm sorry but questions like this reek of trolling. If you have issues, then please cite some science backing your position.
Patrick
Notice also in the summary in the post, the reference to chapter 16, that the key value - Ω - depends on both carbonate ion concentrations and calcium ion concentrations. The paper scaddenp referred you to is discussing issues with calcium ion availability in fresh water and the mussels need to evolve an active pumping mechanism to collect calcium which isn't commonly available in fresh water.
Sea water in contrast has large amounts of calcium in it - thats where all the dissolved minerals end up.
The chemistry of calsification is significantly different between fresh water and sea water.
Aleks,
It appears that you have abandoned your previous arguments. I presume that you accept that not enough NO2 and SO2 are emitted to affect ocean pH and that your calculation was incorrect.
You currently argue that
"The result can not be explained from the point of view of a chemist. I think that the analysis of these data allows to doubt the correctness of the theory that explains the ocean acidity only by the presence of CO2."
You feel that somehow an argument can be made that since you do not understand why the pH changes with depth that contradicts the fact that CO2 controls the pH of the ocean.
I will note that an argument from ignorance is not a scientific argument.
OA is not OK #14 (whose graph is slightly different from the one you provide) states:
"dissolution of calcium carbonate [in the deep ocean] increases the pH slightly (by removing an acid). As depth increases, the pH does not recover to the surface value and this tells us that there must be more respiration (producing acid) than shell dissolution (consuming acid)."
The Pacific ocean water is lower in pH because it is older than the Atlantic ocean water. Over time more CO2 has dissolved, lowering the pH in the Pacific Ocean.
As the OA is not OK has demonstrated, from a chemists point of view it is easy to explain the pH of the deep ocean. Scientists understand the reasons for the composition of the deep ocean waters. Perhaps you should read your references more carefully.
Responding to Aleks from here: (his previous post is just above the linked post)
You said:
"That's why "dry wall plant" you saw is built not from CaSO4, but from CaCO3 with impurities of CaSO3, Ca SO4, Ca(NO2)2, and Ca(NO3)2. It's not a good building material."
The Wikipedia article on drywall states:
"Drywall (also known as plasterboard, wallboard, gypsum panel, sheet rock, or gypsum board) is a panel made of calcium sulfate dihydrate (gypsum)" (my emphasis)
Your claim that dry wall is made from calcium carbonate is incorrect. You make yourself look stupid when you make false claims that can be easily Googled. Calcium carbonate is what is added to convert the SO2 into calcium sulfate. SInce I am a chemistry teacher I know that the Calcium Sulfate from coal power plant scrubbers is especially high in purity and makes the best drywall.
As for your suggestion that:
""The actual value of the mmol of H+ ions formed from 34 mmol of CO2 is about 37 mmol". This is possible only if H2CO3 dissociates completely (??) as a monopritic acid and partly as a diprotic acid. It contradicts the facts established in chemistry."
I will remind youu that I have provided a reference from an expert and I have taught students how to do this calculation for the past ten years. I reviewed the experts calculation and I got the same values he did.
By contrast you are an anonymous guy on the internet who claims to have a degree in Chemistry. You have provided no citations to support your wild claims, only an incorrect calculation.
When a weak acid is dissolved in distilled water most of the acid does not ionize. When the weak acid is dissolved in a buffer, the pH of the buffer determines how much of the acid ionizes. If the pH of the buffer is greater than the pKa of the acid most of the acid ionizes. If the pH is less than the pKa than most of the acid does not ionize.
Since the apparent Ka of carbon dioxide is 4.5 x 10-7, the pKa is about 6.3. Since ocean surface water is about pH 8.2, about 99.5% of the CO2 has ionized. The actual values of % ionization are:
"At typical surface seawater pH of 8.2, the speciation between [CO2], [HCO3−], and [CO3 2−] is 0.5%, 89%, and 10.5%,"
as I previously posted. These are the "facts established in Chemistry" and calculated from the Ka's of Carbonic acid listed previously. Your "calculation" was incorrect because you do not know how to incorporate the pH into the calculation. You must use the pH.
You do not know how to do calculate the % ionization of an acid in a buffer. Why are you bothering to call out scientists who do calculations you do not know how to do?
Michael sweet @76, @77
I did not accept that “not enough NO2 and SO2 are emitted to affect ocean pH”. In any case, the effect of these gases on pH will be much greater than of CO2. Great emission of SO2 during industrial revolution is shown in MA Rodger's post #23 here:
https://www.skepticalscience.com/jellyfish-teach-us-about-climate-change.html#comments
“The Pacific Ocean water is lower in pH because it is older than the Atlantic ocean. One time more CO2 has dissolved lowering the pH in the Pacific ocean”. This explanation contradicts not only the fact that CO2 concentration in the surface layer is approximately the same, but also the elementary logic. CO2 in seawater is in dynamic equilibrium with CO2 in the atmosphere. Partial pressure of CO2 and water surface temperature increased and decreased countless times, so it's impossible to suggest that many millions of years enhanced concentration of CO2 in Pacific left unchanged.
@77. About “dry wall plant”. You said before that this object is made from the material extracted from the scrubber after absorption of gas emission from the coal power plant. I'd like to recall that the absorbent in this case is Ca(OH)2 that reacts not only with SO2, but with CO2 that is the main component of emitted gases, hence, scrubber material consists mainly of CaCO3. This issue is relevant to our main problem, since it is about whether scrubbing can eliminate SO2 and NO2 emissions into atmosphere. Evidently, it can not, because this process is expensive, time and labor consuming and leads to a huge amount of solid waste. Therefore, emissions of acid gases into the atmosphere do not stop, especially in China, India which consume more than half the world's coal production.
I will not discuss your assertion about complete dissociation of H2CO3 in alkaline buffer solution based on “a reference from expert”. There is no specific calculation – there is no discussion. Let's consider real values of CO2 in seawater (table 1.2, p.24) in the work I cited before:
https://www.iaea.org/ocean-acidification/act7/Guide%20best%20practices%20low%20res.pdf The concentrations of carbon containing species in seawater are (micromole /kg): bicarbonate 1718, carbonate 239, dissolved carbon dioxide 9.6. Even assuming that all CO2 converts to H2CO3 and it dissociates completely, amount of [H+] from CO2 is less than from 1mg NO2 converted to HNO3 (~22 micromole [H+]).
Aleks,
Nigelj, MA Rodger and I have shown that all your arguments are false or based on incorrect calculations. It is not our problem if you cannot understand basic facts and data. At this site you have to support your arguments with more than your opinion. You will never convince anyone here.
Recommended supplemental reading:
Melting Ice Could Mess Up Deep Sea Chemistry by Chelsea Harvey, Climate Wire/Scientific American, Nov 28, 2017
Hi, I was wondering why CaCO3 precipitate precisely on plankton and shellfishes. Are they catalizing the formation of CaCO3 (i.e. changing some of the reaction cinetic in some ways) locally? If so, what is the contribution on the overall slow C cycle? Would it "turn" much slower if they weren't there?
PS: Thanks for this amazing website :)
[BL] Please avoid posting the same comment on multiple threads. It leads to a fragmented discussion when people respond.
Readers will find all recent comments on the Comments page. There is a link to that page in the middle of the menu, just below the main header.
mm @81,
The 'plankton and shellfishes' are expending energy pumping ions about, concentrating Ca2+ where it is required** while ejecting H+. I'm afraid the biochemistry of all this pumping is beyond my pay grade (although it could be obtained form them what knows their biochemistry. As an exemplar, consider Calcium ATPase).
** Apparently regulating Ca2+ concentration in biology is a common process, but mainly to keep Ca2+ levels down.