Is the CO2 effect saturated?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
The notion that the CO2 effect is 'saturated' is based on a misunderstanding of how the greenhouse effect works. |
|||||||
Climate Myth...
CO2 effect is saturated
"Each unit of CO2 you put into the atmosphere has less and less of a warming impact. Once the atmosphere reaches a saturation point, additional input of CO2 will not really have any major impact. It's like putting insulation in your attic. They give a recommended amount and after that you can stack the insulation up to the roof and it's going to have no impact." (Marc Morano, as quoted by Steve Eliot)
At-a-Glance
This myth relies on the use (or in fact misuse) of a particular word – 'saturated'. When someone comes in from a prolonged downpour, they may well exclaim that they are saturated. They cannot imagine being any wetter. That's casual usage, though.
In science, 'saturated' is a strictly-defined term. For example, in a saturated salt solution, no more salt will dissolve, period. But what's that got to do with heat transfer in Earth's atmosphere? Let's take a look.
Heat-trapping by CO2 in the atmosphere happens because it has the ability to absorb and pass on infra-red radiation – it is a 'greenhouse gas'. Infra-red is just one part of the electromagnetic spectrum, divided by physicists into a series of bands. From the low-frequency end of the spectrum upwards, the bands are as follows: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Gamma rays thus have a very high-frequency. They are the highest-energy form of radiation.
As our understanding of the electromagnetic spectrum developed, it was realised that the radiation consists of particles called 'photons', travelling in waves. The term was coined in 1926 by the celebrated physicist Gilbert Lewis (1875-1946). A photon's energy is related to its wavelength. The shorter the wavelength, the higher the energy, so that the very high-energy gamma-rays have the shortest wavelength of the lot.
Sunshine consists mostly of ultraviolet, visible light and infra-red photons. Objects warmed by the sun then re-emit energy photons at infra-red wavelengths. Like other greenhouse gases, CO2 has the ability to absorb infra-red photons. But CO2 is unlike a mop, which has to be wrung out regularly in order for it to continue working. CO2 molecules do not get filled up with infra-red photons. Not only do they emit their own infra-red photons, but also they are constantly colliding with neighbouring molecules in the air. The constant collisions are important. Every time they happen, energy is shared out between the colliding molecules.
Through those emissions and collisions, CO2 molecules constantly warm their surroundings. This goes on all the time and at all levels in the atmosphere. You cannot say, “CO2 is saturated because the surface-emitted IR is rapidly absorbed”, because you need to take into account the whole atmosphere and its constant, ongoing energy-exchange processes. That means taking into account all absorption, all re-emission, all collisions, all heating and cooling and all eventual loss to space, at all levels.
If the amount of radiation lost to space is equal to the amount coming in from the Sun, Earth is said to be in energy balance. But if the strength of the greenhouse effect is increased, the amount of energy escaping falls behind the amount that is incoming. Earth is then said to be in an energy imbalance and the climate heats up. Double the CO2 concentration and you get a few degrees of warming: double it again and you get a few more and on and on it goes. There is no room for complacency here. By the time just one doubling has occurred, the planet would already be unrecognisable. The insulation analogy in the myth is misleading because it over-simplifies what happens in the atmosphere.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
This myth relies on the use of a word – saturated. When we think of saturated in everyday use, the term 'soggy' comes to mind. This is a good example of a word that has one meaning in common parlance but another very specific one when thinking about atmospheric physics. Other such words come to mind too. Absorb and emit are two good examples relevant to this topic and we’ll discuss how they relate to atmospheric processes below.
First things first. The effect of CO2 in the atmosphere is due to its influence on the transport of 'electromagnetic radiation' (EMR). EMR is energy that is moving as x-rays, ultraviolet (UV) light, visible light, infrared (IR) radiation and so on (fig. 1). Radiation is unusual in the sense that it contains energy but it is also always moving, at the speed of light, so it is also a form of transport. Radiation is also unusual in that it has properties of particles but also travels with the properties of waves, so we talk about its wavelength.
The particles making up radiation are known as photons. Each photon contains a specific amount of energy, and that is related to its wavelength. High energy photons have short wavelengths, and low energy photons have longer wavelengths. In climate, we are interested in two main radiation categories - firstly the visible light plus UV and minor IR that together make up sunshine, and secondly the IR from the earth-atmosphere system.
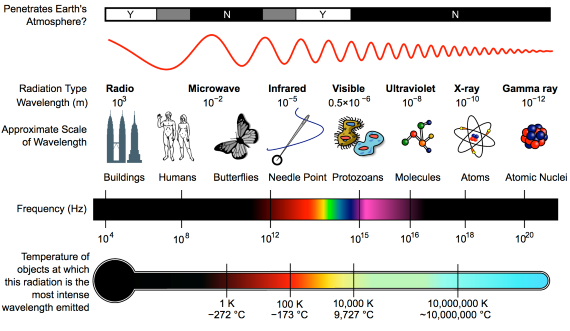
Fig. 1: diagram showing the full electromagnetic spectrum and its properties of the different bands. Image: CC BY-SA 3.0 from Wikimedia.
CO2 has the ability to absorb IR photons – it is a 'greenhouse gas'.So what does “absorb” mean, when talking about radiation? We are all familiar with using a sponge to mop up a water spill. The sponge will only absorb so much and will not absorb any more unless it's wrung out. In everyday language it may be described, without measurements, as 'saturated'. In this household example, 'absorb' basically means 'soak up' and 'saturated' simply means 'full to capacity'. Scientific terms are, in contrast, strictly defined.
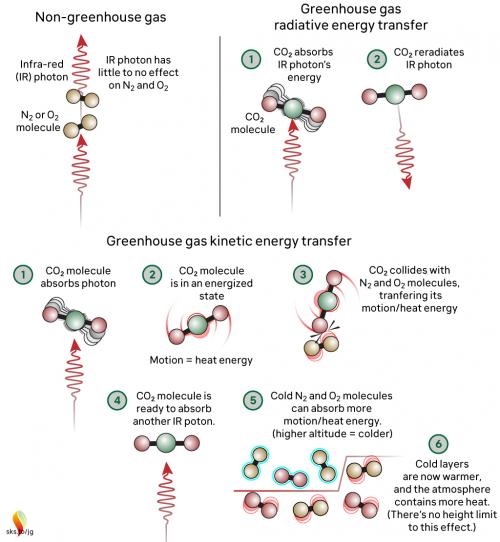
Now let's look at the atmosphere. The greenhouse effect works like this: energy arrives from the sun in the form of visible light and ultraviolet radiation. A proportion reaches and warms Earth's surface. Earth then emits the energy in the form of photons of IR radiation.
Greenhouse gases in the atmosphere, such as CO2 molecules, absorb some of this IR radiation, then re-emit it in all directions - including back to Earth's surface. The CO2 molecule does not fill up with IR photons, running out of space for any more. Instead, the CO2 molecule absorbs the energy from the IR photon and the photon ceases to be. The CO2 molecule now contains more energy, but that is transient since the molecule emits its own IR photons. Not only that: it's constantly colliding with other molecules such as N2 and O2 in the surrounding air. In those collisions, that excess energy is shared with them. This energy-sharing causes the nearby air to heat up (fig. 2).
Fig. 2: The greenhouse effect in action, showing the interactions between molecules. The interactions happen at all levels of the atmosphere and are constantly ongoing. Graphic: jg.
The capacity for CO2 to absorb photons is almost limitless. The CO2 molecule can also receive energy from collisions with other molecules, and it can lose energy by emitting IR radiation. When a photon is emitted, we’re not bringing a photon out of storage - we are bringing energy out of storage and turning it into a photon, travelling away at the speed of light. So CO2 is constantly absorbing IR radiation, constantly emitting IR radiation and constantly sharing energy with the surrounding air molecules. To understand the role of CO2, we need to consider all these forms of energy storage and transport.
So, where does 'saturation' get used in climate change contrarianism? The most common way they try to frame things is to claim that IR emitted from the surface, in the wavelengths where CO2 absorbs, is all absorbed fairly close to the surface. Therefore, the story continues, adding more CO2 can’t make any more difference. This is inaccurate through omission, because either innocently or deliberately, it ignores the rest of the picture, where energy is constantly being exchanged with other molecules by collisions and CO2 is constantly emitting IR radiation. This means that there is always IR radiation being emitted upwards by CO2 at all levels in the atmosphere. It might not have originated from the surface, but IR radiation is still present in the wavelengths that CO2 absorbs and emits. When emitted in the upper atmosphere, it can and will be lost to space.
When you include all the energy transfers related to the CO2 absorption of IR radiation – the transfer to other molecules, the emission, and both the upward and downward energy fluxes at all altitudes - then we find that adding CO2 to our current atmosphere acts to inhibit the transfer of radiative energy throughout that atmosphere and, ultimately, into space. This will lead to additional warming until the amount of energy being lost to space matches what is being received. This is precisely what is happening.
The myth reproduced at the top – incorrectly stating an analogy with roof insulation in that each unit has less of an effect - is misleading. Doubling CO2 from 280 ppm to 560 ppm will cause a few degrees of warming. Doubling again (560 to 1130 ppm) will cause a similar amount of additional warming, and so on. Many doublings later there may be a point where adding more CO2 has little effect, but recent work has cast serious doubt on that (He et al. 2023). But we are a long, long way from reaching that point and in any case we do not want to go anywhere near it! One doubling will be serious enough.
Finally, directly observing the specific, global radiative forcing caused by well-mixed greenhouse gases has - to date - proven elusive. This is because of irregular, uncalibrated or limited areal measurements. But very recently, results have been published regarding the deep reinterrogation of years of data (2003-2021) from the Atmospheric Infrared Sounder (AIRS) instrument on NASA's Aqua Satellite (Raghuraman et al. 2023). The work may well have finally cracked the long-standing issue of how to make finely detailed, consistent wavelength-specific measurements of outgoing long-wave radiation from Earth into space. As such, it has opened the way to direct monitoring of the radiative impact (i.e. forcing + feedback) of greenhouse gas concentration changes, thereby complimenting the Keeling Curve - the longstanding dataset of measured CO2 concentrations, down at the planet's surface.
Note: Several people in addition to John Mason were involved with updating this basic level rebuttal, namely Bob Loblaw, Ken Rice and John Garrett (jg).
Last updated on 31 December 2023 by John Mason. View Archives































 Arguments
Arguments





































fred.steffen... I did a quick google on the guy who apparently wrote the article for Goddard's blog. He says his name is Mike Sanicola, and he states that he is a "professional IR astronomer." In my google search I came up with this person who also checked into Sanicola's credentials. He says:
So, I don't know who this guy actually is but he's clearly not the expert he claims to be.
fred.steffen: I don't know what you mean by radiative forcing not fitting as a model. Nor do I know what other model you claim is used by most climate models, so please explain.
A response to that article you linked does a good job debunking that article, including the nonexistence of the supposed IR astronomer. However, I'd steer clear of its link to WUWT. Instead click the link on the Pierrehumbert article. For a more accessible explanation, see the Skeptical Science post "How Do We Know More CO2 Is Causing Warming?"--first watch the video at the bottom, then read the Basic tabbed pane, then the Intermediate tabbed pane, then the Advanced one.
The article on Tony Heller's (AKA Steven Goddard) states:
Starting at 13 we get CO2 absorption but that wavelength corresponds to temperatures below even that of the south pole.
So whoever he is, he doesn't seem to understand the earths emission spectrum (as a pseudo-black body) but appears to think the earth should emit at a single frequency for each "parcel" of the surface that is at a particular temperature.
Fred - so you would happily breath air with 0.04% Hydrogen Cyanide? Trying to dismiss an effect because it is a small number with without doing the maths to see if what effect it really has is more like an argument from Personal Incredulity. Does it sound better if you say the CO2 makes up 60% of the radiatively active gases in the atmosphere?
Also, you suggest experiments to see the effect of CO2 - but this kind of thing has already been done. See the Advanced tab of Tom Dayton's link for the papes. The effect of CO2 on incoming radiation has been measured from the surface and also the effect on outgoing from satellite. Both measurements agree with theoretical model to a very high degree of precision. A direct measurement has also been achieved. See here.
fred.steffen @399:
1) when you say "as a model, [radiative forcing is] not fitting", the model from which radiative forcing is derived are Line By Line (LBL) or broadband radiative models. The Line By Line refers to the fact that they calculate atmospheric transmission and emission for each wave number (a measure of frequency) seperately, giving a very fine resolution of radiative transfer. Typically they also divide the amtosphere into about twenty layers or so, calculating in each direction (up or down) the radiation entering, the radiation absorbed and the radiation emitted based on the atmospheric composition at that layer. As of 1969, they produced results with this sort of accuracy:
One such model whose accuracy across a wide range of surface conditions, temperatures and latitudes was studied in 2008 showed the following scatter plot vs observations for 134,862 observations:
If you are not familiar with scatter plots, they are plots of the observed value (CERES OLR) with the model predicted value, with perfect accuracy of prediction meaning the observations sit on the black line shown. The accuracy shown here is absolutely astonishing. The determination of radiative forcing of CO2 was done using models like this, or the lower resolution versions that are essential parts of all climate models (Global Circulation Models). I can only presume that when you say the model is "... not fitting", you simply do not know what models are used for the theory.
2) You also say that "If CO2 makes up 20% of our greenhouse effect, light from stars at this wavelength should be diminished by 20%". That assumes that absorption is the same at all frequencies, which is false (as can be seen in the first graph). IR astronomers tune the frequency of the observatories to 10 to 13 micron (800 - 1000 cm-1)band where there is minimum absorption by any atmospheric component as seen in the first grap above, and this emission spectrum from the University of Colorado:
By doing so they avoid nearly all of the effect of CO2 and H2O on the incoming light. Despite this, they still need to place their observatories high in the atmosphere (either on mountains, in planes or supported by balloons) or in space to get clear images. So, your fundamental premise that absorption is equal across all IR bands is simply mistaken.
Curiously, Goddard's "IR astronomer" friend refers to the 9.5 micron band as being absorption freed (it is in fact the frequency of maximum absorption and emission by ozone) and describes the actual atmospheric window as being a zone of significant absorption and emission by H2O, showing he does not even grasp the fundamental facts of atmospheric absorption and emission.
3) "Steven Goddard" and his (apparently fictional) source always makes a fundamental misake in examining radiation models. He only examines the so-called back radiation. Because H2O and CO2 emissions overlap, and because H2O is very abundant in the low atmosphere, CO2 emissions make up only a very small percentage of the overall back radiation. That, however, is irrelevant. What controlls the Global Mean Surface Temperature (GMST) is the balance of energy recieved and energy radiated to space. Therefore it is radiation to space from the atmosphere which is the dominant driver of surface temperatures, and hence upper atmosphere concentrations that matter. Because the concentration of H2O is controlled by temperature, and temperatures fall rapidly with altitude, CO2 completely dominates emission to space in frequencies of significant overlap with H2O. Consequently, it is emissions to space that must be examined to determine the relative importance of different atmospheric components.
As an aside, because H2O absorbs in more frequencies it still (along with clouds) accounts for 75% of the total greenhouse effect, with CO2 accounting for 20%. Importantly, H2O varies rapidly with surface temperature, while CO2 varies only slowly. As a result, increasing CO2 will result in a rapid rise in H2O, generating a positive feedback on the CO2 rise. In contrast, a rise in H2O will result in only a small response from CO2, resulting in temperatures and H2O concentrations soon returning to their initial values.
Finally, if you want to examine the basis of greenhouse effect in more detail, but explained very clearly, I recommend my post here. It and the following comments also contain more detail on the first two graphs above.
(Note to the moderator, I know that I am close to the point of dogpilling. If that is a problem, I ask that you retain my post as the only one todate directly addressing the issues raised by fred.steffen (rather than his sources). Thankyou)
[PS] Tom, thank you for your considered and detailed response. I am sure that will be helpful. However, this is close to dogpiling so no more please.
Rob Honeycutt:
Mike Sanicola is Morgan Wright. He runs the Hyzer Creek Disk Golf course in New York. His YouTube ID is hyzercreek. "Mike Sanicola" is actually the name of a baseballer who was drafted to the Yankees in 1983, was seriously injured, then went into the ministry.
As Morgan Wright, he claims to be an optometrist. In his most recent YouTube comments, he made the mistake of mixing the two identities, which tipped me off to the Sanicola identity.
He says "I'm an opticist, who specializes in optics and IR. I worked for GE's infrared department and designed infrared telescopes for GE that were used by NASA in outer space. I invented the ambient temperature microbolometer."
Also, no proof yet, but I am beginning to wonder if he is actually Steven Goddard. He regularly links to Goddard's site, and their manner of speech and desire to advertise dual occupations are eerily similar.
so, where is the answer to the article ?
the curve relating the CO2 concentration in atmosphere and temperature of lower atmosphere ?
it should be a simple thermodynamic equilibrium wich depends on absorption and emittance parameters,
and yes even with 100% of CO2 the temperature would be finite (because of the Stefan-Boltzmann black body rule which says the irradiance depends of temperature in T4 : the more a body is hot, the more it cools itself by irradiating around)
Can anyone explain how adding CO2 causes a supposed emission from higher altitude and therefore a higher surface temperature? The suggesiton isnt that the earths atmosphere became thicker, with a vertical lapse rate movement, the suggection is that if an emitted photon is now picked up and re-emitted from 100 meters further up this photon has lost energy, and that energy has somehow got back to the surface.
Can anyone explain this to me?
ConcernedCitizen @408, I am sure that nobody can explain it to you, as you have no interest in learning. That, at least, is evident from the straw man you construct. The actual theory is that CO2 in the tropophere at altitude z has less thermal energy than CO2 at altitude z-100 meters. Therefore the total thermal emissions by CO2 at altitude z will be less than that by CO2 at altitude z-100 meters. Individual photons will have approximately the same energy (which is a function of wavelength), but fewer of them will be emitted.
As to why the CO2 has less thermal energy with altitude, a partial answer is that because it is at a higher altitude, it has more gravitational potential energy and less energy of motion (which includes thermal energy). That relationship is further modulated by changes in pressure with altitude (decreasing pressure cools gasses). If you want to know more, you can start reading here.
In your incoherent way, you are actually denying a combination of the first law of thermodynamics, the ideal gas laws, Newton's law of gravitation, and Planck's law. Not a bad score in the science denial stakes.
TomCurtis @409. Putting your insults to one side, what you are talking about it kinetic to radiative change in energy. However, if the CO2 molucule wasnt there, the energy wouldnt even radiate, it would stay as kinetic and remain in the atmosphere.
(This cooling effect of GH gasses at low pressures by kinetic->radiative change is well understood)
Now, if you can answer the question ina civilised maner it would be appreciated.
[PS] Civility all round would be appreciated. CC it also behoves on you to study the answers and resources suggested to you for understanding if you are asking questions, otherwise people quickly lose patience. If you have not read Ramanathan and Coakley 1978 I suggest you do so and make it clearer whether you contest the Radiative Transfer Equation fundamentals or their particular application in discussions with people here.
ConcernedCitizen @410.
You say "If the CO2 molucule wasn't there, the energy wouldn't even radiate. It would stay as kinetic and remain in the atmosphere." That's a bit tricky. (I'm reminded of the rhyme ".../He wasn't there again today/I wish that man would go away." )
So if the CO2 molecule isn't there, where exactly in the atmosphere is this kinetic energy you speak of and how did it get there?
ConcernedCitizen @410, ignoring clouds, the consequence of there being no GHG in the atmosphere (not just CO2) is that there is no IR radiation to space from the atmosphere; but also no absorption by the atmosphere of surface IR radiation. The consequence can be seen in the surface energy balance diagram below:
Specifically, because there is no IR absorption in the atmosphere by your scenario, the 397 W/m^2 IR radiation from the surface would escape to space. On the other hand, there would be no 342 W/m^2 back radiation (thermal down surface), so the incoming energy would be only 240 W/m^2 (Incoming solar minus solar reflected). The resulting energy imbalance of -157 W/m^2 would result in very rapid cooling until the IR radiation from the surface matched the incoming solar (ie, to - 18 C).
Atmospheric IR emission is not a "cooling effect". You can only think it is because you do not take into account all of the related energy exchanges.
M A Roger @411
Tom Curtis @412
The quesiton isnt whether the GH effect exists, it is wether CO2 is saturated as GH gas.
The suggesiton is this: A CO2 mollucule at 6 km radiates a photon upwards. This photon was either radiated out from the surface and stayed as a photon at 15 microns all the way up, and finally, this CO2 molecule was the last one in the chain, and the photon made it out to space.
Or the CO2 mollecule was impacted by an O2, or N2 molecule and thus kinetic to radiative change happened, with the energy of the photon representing the energy at that altitude, ie, temperature.
The proposal here is that an additional CO2 mollucule at 6.1 km causes cooling. HOw so? Lets look at the wto cases. Either the photon was passed up from the surface, and this is the last CO2 mollecule in the chain, in which case it is pased out to space with exactly the same energy as before. Ie, no loss of energy, no warming.
Or, if the photon came from kinetic energy and was radiated out to space, then yes, the energy of the photon would be less, because it represents the temperature at 6.1 km, but if the CO2 mollecule werent there then this radiation wouldnt happen at all and the kinetic energy would have just stayed in the system.
So I dont see how the suggesiotn that 'A CO2 mollecule radiates from higer and colder' means additional energy in the system and hence warming.
ConcernedCitizen @413:
You say that, but immediately mount an argument that, if valid, would mean there is no greenhouse effect, not that it was saturated.
In fact, both of these are true for very small parts (<1% at a guess) of the energy radiated to space by CO2 from the middle to upper troposphere. For most energy, it will have been radiated from the surface (68%), or transferred by latent heat or conduction (18%), or absorbed in the atmosphere from solar radiation (14%). From there, most energy transfers will have been by collisions with other molecules, with transfer to molecules travelling downwards as likely as those to molecules travelling upwards, and with lateral motion of molecules receiving energy as great as either upwards or downwards motion. Most upwards motion will be from emissions from molecules, but (firstly), radiation will be as likely to take energy downwards as up, equally likely to take it laterally as either; and the molecules radiating the energy are more likely to be H2O molecules in the lower troposphere than CO2 molecules (and hence have a different, but lower energy content per photon than that eventually radiated to space by CO2). Of course, some of the radiation will have been by radiated by other greenhouse gases (CH4, NO2, O3, etc) which typically have a higher energy content per photon than that radiated by CO2. Of course, energy radiated by a greenhouse gas other than CO2 will have to be absorbed by that same greenhouse gas (except for a small amount radiated by H2O) and then transferred to CO2 by collisions (probably mediated by collisions with N2 and O2). Even energy transferred by radition from CO2 and absorbed by CO2 will have different energies to that finally radiated to space because of doppler energy shifts, and pressure broadening (and a couple of other effects).
The net consequence of this is that:
1) Of the energy finally radiated to space by a single CO2 molecule, not all will have come by the same pathway except in very rare cases;
2) The energy finally radiated by CO2 to space will typical have followed a very convoluted pathway through the atmosphere, spending almost as much time travelling downwards as upwards, and twice as much time travelling laterally as either downwards or upwards (with different parts of the energy travelling different paths as per (1));
and (most importantly)
3) Because the lower layers of the atmosphere are warmer, and hence radiate more energy, and downward radiation is as probable as upward radiation at all levels of the atmosphere, the majority of energy entering the atmosphere (59%) will follow a convoluted path that returns it to the Earth's surface.
The upshot is that the convoluted pathway of energy from the surface introduced by the presence of greenhouse gases in the atmosphere serves as a filter limiting the escape of energy to space. There is a bias in that filter caused by the lower temperatures at higher altitudes which means that less than 50% of the energy escapes to space.
Unfortunately for your theory, none of this fits your simplistic analysis. In your analysis, either all radiation is upward only, in which case all energy emitted from the surface escapes to space without impediment. And indeed, if all radiation was upwards only, there could be no greenhouse effect - but such a situation is unphysical.
Alternatively, on your second scenario, energy is transfered upwards by collisions and IR radiation from the upper troposphere reduces the thermal energy relative to the case with no greenhouse gases: but you neglect that the lack of greenhouse gases also reduces the introduction of energy into the atmosphere by absorption of thermal radiation from the surface, with the absorbed IR radiation from the surface being much greater than the emitted radiation from the upper atmosphere. So, while the IR radiation to space cools the atmosphere, the IR absorption from the surface warms it at a much faster rate.
In both scenarios, you ignore essential features of the system in order to draw obtuse conclusions.
[PS] Thanks Tom. ConcernedCitizen, note that the effects of increased CO2 has been directly measured (see "Observational determination of surface radiative forcing by CO2 from 2000 to 2010") so it real despite your difficulties in understanding why.
Tom Curtis@414
Not wanting to create a long thread, and to keep it trelevant to the theory postulasted here, I focused on heat transfer space, not from the surface and not internally, so it wasnt a refutation of the GH effect at all.
" but you neglect that the lack of greenhouse gases also reduces the introduction of energy into the atmosphere by absorption of thermal radiation from the surface, with the absorbed IR radiation from the surface being much greater than the emitted radiation from the upper atmosphere"
At the top of the atmosphere, where the additional CO2 mollecules are now radiating at higher altitude, you have said that less than 1% of energy is direct radiation from the surface which contradicts this statement.
"if we add more greenhouse gases the air needs to be thinner before heat radiation is able to escape to space"
Why? If it isnt direct radiation from the surface (in which case the energy is maintained regardless of what altitude its radiated at, and is very rare (free path lenght and all that)) then its a kinetic-radiative change of energy.
So, if a colision happens with CO2 at 6km and is radiated to space the photon's energy represents the temperature at 6km.
Add more CO2 and now more of the collisions happen at 6.1km with the photon's energy representing the temperature at that altitude.
This doesnt mean there is more heat in the system.
Better to say that adding more CO2 turns kinetic energy at 6.1 km into photons which have an even chance of being emitted towards the surface. ie, more GH gas = more back radiaiton.
Taking the Feldmanpaper data from the paper above, and using 370 and 390 ppm for CO2 at 2000 and 2010 MODTRAN gives a 0.3 wm^-2 forcing change, which isnt far off fro the Feldman value, so I am not doubting CO2 as a GH gas or CO2 non-saturation just that the mechanism proposed here isnt feasible.
Add CO2 and now we have
Look, I am not saying CO2 is saturated, but the mechanism proposed here doesnt hold water when you pull it apart, and you certainly havent explained it, all you have done is explain the GH effect.
[RH] Removed excess blank space. Concerned Citizen, the process has been explained to you in a very detailed and accurate manner. You're skating on this ice at the moment.
Please note that posting comments here at SkS is a privilege, not a right. This privilege can be rescinded if the posting individual treats adherence to the Comments Policy as optional, rather than the mandatory condition of participating in this online forum.
Please take the time to review the policy and ensure future comments are in full compliance with it. Thanks for your understanding and compliance in this matter.
Link to the commenting policy is just above the comment window.
[PS] The detailed process with the maths is in the Ramanathan and Coakley paper pointed to earlier. Do you dispute that paper?
Frankly ConcernedCitizen has amply demonstrated his refusal to think clearly about this topic. I am no longer going to waste my time on him. When he is so wrapped up in his own "wisdom" that he does not recognize the air at 6.1 km is thinner than that at 6.0 km, and that consequently his own counterexample proves the claim he is disputing, it is completly pointless to continue any discussion. (Romans 1:22 applies.)
@Moderator: Right, so when I point out that I accept that CO2s GH effect isnt saturated but question the proposed explanation I am going to be blocked am I?
Tom Curtis@416
Air at 6,1 km is thinner, I now that, I already said its colder. How does putting an additional CO2 mollecule there cause warming? By changing kinetic into radiative energy and radiating it downwards.
The suggestion that there is a kind of 'CO2 lapse rate' just doesnt fit.
[JH] Moderation complaint snipped.
Please note that posting comments here at SkS is a privilege, not a right. This privilege can and will be rescinded if the posting individual continues to treat adherence to the Comments Policy as optional, rather than the mandatory condition of participating in this online forum.
Moderating this site is a tiresome chore, particularly when commenters repeatedly submit offensive or off-topic posts. We really appreciate people's cooperation in abiding by the Comments Policy, which is largely responsible for the quality of this site.
Finally, please understand that moderation policies are not open for discussion. If you find yourself incapable of abiding by these common set of rules that everyone else observes, then a change of venues is in the offing.
Please take the time to review the policy and ensure future comments are in full compliance with it. Thanks for your understanding and compliance in this matter.
Concerned Citizen,
It is too bad that you have alienated Tom Curtis. He generally is patient with learners and provides lots of data to support the consensus.
You need to step back and address one of your misconceptions at a time. When you list 5 or 6 misconceptions in a single post it is difficult to respond without a major post. You need to read the OP again and see what you misunderstand.
The increase in the radiation altitude causes the Earth to warm because of the temperature lapse rate. As you go higher in the atmosphere it gets colder. When the emission altitude is increased, less energy is emitted because it is colder. In order to reach equilibrium, the temperature must increase at 6.1 km so that the emitted energy equals the energy incoming from the Sun. Because the temperature lapse rate in the atmosphere stays the same, in order for the temperature to increase at 6.1 km, the surface has to warm also.
If you still do not understand why increasing emission altitude causes the surface to warm post again here. Do not add additional questions until we establish this description of the increase in temperature.
This is NOT the correct explanation of the saturation of CO2 absorption of IR radiation. The fact is that the effect IS saturated at the resonant frequency of bending of a CO2 molecule (wave number 667).
However molecules CAN absorb radiation at frequencies that don't match the resonant frequency, albeit with a lower probability of absorption.
IR radiation at the resonant frequency has a mean free path of just over 30 cm with the current CO2 concentration. So almost all of that radiation makes it back to the ground. In fact that frequency becomes saturated at only a couple of parts per million.
A rough rule of thumb is that the probability of absorption reduces to about 10% for every 5% change in IR frequency. So the mean free path is multiplied by 10. So IR radiation which differs in frequency from the resonant frequency by 20% has a mean free path of about 3 km. At these altitudes the calculation is complicated by the thinning atmosphere, so the mean free path would actually be somewhat longer than that.
The effect of adding more CO2 is to reduce the mean free path of IR radiation at ALL frequencies. This has the effect of widening the band of frequencies that attain absorption saturation.
This is called BAND SATURATION. It is the widening of the band of frequencies that achieve saturation that causes the greenhouse effect to increase as more CO2 is added. And mathematically, it explains why the temperature effect increases logarithmically with CO2 concentration (ie. a constant temperature increase for every doubling of CO2 concentration).
I strongly suggest you change your article to reflect this science.
[RH] All caps is against policy. Please take the time to read our commenting policies before you continue.
braintic @419, thankyou for your clear, and accurate exposition of the band saturation effect. That is a genuine effect that is described very clearly by David Archer in Chapter 4 of his book, Climate Change: Understanding the Forecast (available online here); and also by Riccardo in the advanced version of the rebutal to the myth. The reason band saturation is presented as the advanced rebutal, while increased effective altitude of radiation is presented as the rebutal of the "CO2 is saturated" myth is that band saturation is a subtle consequence of the fundamental effect of the increasing altitude of radiation in an atmosphere in which the stratosphere warms with altitude (something I will explain in more detail below). In an atmosphere with a cooling stratosphere with altitude, there would be no band saturation - and the greenhouse effect would be stronger as a result.
You are clear that increasing CO2 causes increasing warming, but you appear to miss the fact that the myth is not about band saturation, but rather the myth is a claim that CO2 is saturated across all bands, and that this means increasing CO2 concentration will cause no warming. Given that Skeptical Science targets its basic explanations to those who have studied no science since their 10th year of education, you will understand that a basic rebutal saying that "CO2 is band saturated, but not saturated" is likely to generate confusion. Hence, the basic rebutal concentrates on the fundamental mechanism, while more advanced rebutals deal with the more subtle effects and refer those who are interested to more detailed explanations.
In any event, the theory of the greenhouse effect is fundamentally a theory about the energy balance between the Earth and space (including radiation from the Sun). It follows that the relevant altitude at which to determine "saturation" is not from the surface, but from space (effectively 70 km altitude). At 70 km, the mean free path for the resonant frequency of CO2 is not just over 30 cm, but effectively infinite in a tangent to the Earth, or approximately 35 Km vertically towards the Earth's surface. Adding more CO2 to the atmosphere decreases that downward, vertical mean free path, and hence the distance IR radiation from the Sun can penetrate the atmosphere from space. In more standard terms for climate science, adding CO2 reduces the optical depth of the atmosphere. (Note that I am not saying "mean free path" and "optical depth" are the same thing. They are not. But they are related concepts, and the later is most commonly used in discussing the issue in climate science.)
Because the mean free path downward from space has decreased at the resonant frequency, by Kirchoff's Law the mean altitude of radiation to space increases. That is because, altitude determined by mean free path downward is also the mean altitude of radiation to space, but while the former is measured from the notional boundary to space, the later is measured from the surface. Further, the same effect will be experienced at all frequencies in which CO2 absorbs IR radiation, and therefore also over the average of all thermal IR frequencies. Thus, when speaking of the effect of adding CO2, a simpler way of saying what happens is to say:
Of course, while it is generally true that as we get higher in Earth's atmosphere, it gets cooler, it is not true in either the tropopause or the stratosphere:
That means that for those frequencies of CO2 absorption where the mean free path from the nominal edge of space places the mean level of radiation to space in the tropopause, increasing CO2 will not change the temperature of the gas radiating to space, and therefore not change the energy radiated to space at that frequency. At the resonant frequency of CO2, the mean altitude of radiation to space is in the stratosphere, so that increasing CO2, all else being equal, would result in more radiation to space. As it happens, not all else is equal and that excess CO2 cools the stratosphere on a very short time scale (hours) so in practise the effect is minimal change in the radiation to space. At the wings of the CO2 absorption band, however, the mean altitude of radiation to space is in the troposphere. Consequently raising the mean altitude of radiation to space decreases IR energy transmitted to space at that frequency until the mean altitude of radiation to space reaches the tropopause.
It follows from this that the band saturation effect represents a saturation of the energy radiated to space as the mean altitude of radiation to space enters the tropopause for given frequencies. It does not represent optical saturation (as it would need to be relevant to the myth). Rather, it represents a stable level of radiation to space due to the mean altitude of radiation to space at a given frequency being in the tropopause.
It should be noted that H2O (due to precipitating out rapidly with increased altitude) and other greenhouse gases (due to relatively low concentration) do not have this effect. Their mean altitude of radiation is firmly in the troposphere and increasing concentrations do not lead to band saturation for that reason. The one exception is stratospheric ozone, and stratospheric H2O (introduced by jet fuel). Because both are in the stratosphere, increased altitude increases thermal radiation to space.
michael sweet @418 Yes, I know about the temperature lapse rate, didnt 'Air at 6,1 km is thinner, I now that, I already said its colder' make that self evident?
So far not one of you has been able to describe the physics, the mechanical process, whereby putting an additional CO2 mollecule at 6.1 km raisies temperature by interaction wioth the lapse rate.
The lapse rate is based on atmospheric pressure, so if you added enough CO2, yes, you would increase the atmorpheric pressure, but this isnt the suggestion here and certainly isnt hapening because atmopspheric pressure isnt increasing.
So again, in the absence of any logical and clear explanaiton the explanation given here holds no water.
[JH] If you cannot be civil, you will forfeit your privilege of posting comments on this site.
Concerned Citizen,
The temperature lapse rate is the amount the temperature changes as you increase altitude. It is about 6C per kilometer. That means that if you increase in altitude by one kilometer the air is 6C cooler.
Essentially all the energy emitted by the Earth is at the 6.0 km altitude. This amount of energy equals the amount of energy that arrives each day from the sun. If you increase the amount of CO2 in the atmosphere than the CO2 absorbs more of the energy coming up from the surface. The emission height raises to 6.1 km. This we have agreed on.
Heat is emitted from all materials in amounts proportional to the temperature. When the emission height increases to 6.1 km, less energy is emitted because the air is colder. This is because of the temperature change, not the change in pressure. There is an imbalance between what is arriving from the sun and what is emitted. This imbalance causes the Earth to heat up.
In order to reach a new equilibrium where the heat emitted from the atmosphere is the same as the heat arriving from the sun the temperature at 6.1 km has to increase. Because the lapse rate remains the same, the temperatue of the entire air column under 6.1 km increases the same amount as it increases at 6.1 km. Since the lapse rate is 6C per km the temperature has to increase about 0.6 C if the emission altitude increases from 6.0 to 6.1 km. When the rest of the air column increases in temperature the surface is 0.6C warmer. This is the global warming caused by the increase in CO2.
Do you understand the way that the increase in the emitting altitude (caused by the increase in CO2) results in an increase in the surface temperature?
The atmospheric pressure does not increase measurably from the increase in CO2. This is well known and no-one has suggested that atmospheric pressure changes the temperature. This is an example of a basic misunderstanding that needs to be corrected.
michael sweet @422, I strongly suspect your attempt to answer Concerned Citizen's question will be fruitless. The evidence strongly suggests he is interested in obfustication rather than learning. That is clear from his continuous changing of the question he asks, and he refusal to accept any response as accurate or relevant (despite the responses to his questions having been both).
In attempting to answer Concerned Citizen, however, you stated that "Essentially all the energy emitted by the Earth is at the 6.0 km altitude". That is not correct. The "effective mean altitude of radiation" is approximately 6 km, but the effective mean altitude of radiation is just that altitude in the troposphere at which the globally averaged temperature is the same as the brightness temperature of the IR radiation from Earth, averaged globally and across all frequencies. That that is not the same as the altitude from which essentially all radiation is radiated is most easilly seen by comparing the 6 km temperature to brightness radiation of emission from real observations (in this case averaged over April to June from the Central Pacific for two seperate years, see Harries et al, 2001)
Note that the spike at 1050 cm^-1 is from stratospheric ozone, and is at a much higher altitude than other emissions shown. The central spike from CO2 emissions at 667 cm^-1 is not shown as the graph cuts of at 700 cm^-1.
Just looking at brightness temperatures, unfortunately, will give a mistaken impression as to the effective mean altitude of radiation, as brightness temperatures do not show the intensity of radiation at different wave numbers. For that we need a more traditional emissions spectrum:
In this case we have two such spectra, a clear sky spectrum and one from a thunderstorm anvil. Many cloud spectra will be from lower altitudes. It can be seen, however, how the combination of maximum intensity of surface emission near the CO2 absorption band, combined with the effects of clouds result in an "effective mean altitude of radiation" at around 6 km.
I am fairly certain you knew all this already, but your choice of words leads to an easy misunderstanding, and IMO one that would create confusion if not clarrified.
Tom,
I was hoping that if I made an error that you would step in and correct it. Your summary is correct.
As I understand it, there is a great deal of variation in how radiation gets into space Your post above at 412 shows a small atmospheric window where some IR gets through the atmosphere from the surface. Other wavelengths have different efective mean altitudes of emission depending on how efficiently they are absorbed/radiated by the active molecules. The tropics is different from the Arctic and desert is different from the ocean covered areas. Your post includes all these details.
I meant the "essentially" to summarize all that information and your post at 423 into a single sentence. For the most basic explaination of the greenhouse effect if we assume that all radiation is emitted at 6 km than the explaination of increasing emission height affecting surface temperature is simplified. After one uderstands the basics of how temperature increases due to increasing emission height than the additional details you relate in 423 can be added.
You may be correct that the details are required, but I think it helps to understand the basics by simplifing the complex details. I think here we only differ in what we think is the proper way to simply explain how the greenhouse effect works (and your posts have much better graphics than mine). For more advanced readers (who read but do not comment) your post gives them additional detail so that they understand the effect better than my simplification.
Following up my earlier question regarding CO2 isotopomers and absorption saturation, I get the idea that IR absorption is saturated from, among other sources, the article above "Consider the CO2 absorption band around 15 μm (about 650 cm-1), it is strong enough to not let any light go through after a few tens of meters at surface temperature and pressure." This is not saying the CO2 effect is saturated - there are good arguments and evidence that it isn't - but I am wondering if the minor isotopomers' IR and lower-energy absorption fall within or outside the spectrally-saturated bands. Or perhaps I should use "opaque" instead of "saturation" when asking about absorption spectral lines/bands?